Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4)
- PMID: 23933118
- PMCID: PMC4082531
- DOI: 10.1016/j.mce.2013.08.002
Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4)
Abstract
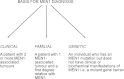
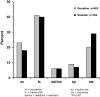
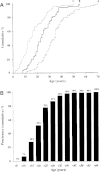
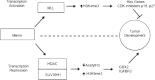
Multiple endocrine neoplasia (MEN) is characterized by the occurrence of tumors involving two or more endocrine glands within a single patient. Four major forms of MEN, which are autosomal dominant disorders, are recognized and referred to as: MEN type 1 (MEN1), due to menin mutations; MEN2 (previously MEN2A) due to mutations of a tyrosine kinase receptor encoded by the rearranged during transfection (RET) protoncogene; MEN3 (previously MEN2B) due to RET mutations; and MEN4 due to cyclin-dependent kinase inhibitor (CDNK1B) mutations. Each MEN type is associated with the occurrence of specific tumors. Thus, MEN1 is characterized by the occurrence of parathyroid, pancreatic islet and anterior pituitary tumors; MEN2 is characterized by the occurrence of medullary thyroid carcinoma (MTC) in association with phaeochromocytoma and parathyroid tumors; MEN3 is characterized by the occurrence of MTC and phaeochromocytoma in association with a marfanoid habitus, mucosal neuromas, medullated corneal fibers and intestinal autonomic ganglion dysfunction, leading to megacolon; and MEN4, which is also referred to as MENX, is characterized by the occurrence of parathyroid and anterior pituitary tumors in possible association with tumors of the adrenals, kidneys, and reproductive organs. This review will focus on the clinical and molecular details of the MEN1 and MEN4 syndromes. The gene causing MEN1 is located on chromosome 11q13, and encodes a 610 amino-acid protein, menin, which has functions in cell division, genome stability, and transcription regulation. Menin, which acts as scaffold protein, may increase or decrease gene expression by epigenetic regulation of gene expression via histone methylation. Thus, menin by forming a subunit of the mixed lineage leukemia (MLL) complexes that trimethylate histone H3 at lysine 4 (H3K4), facilitates activation of transcriptional activity in target genes such as cyclin-dependent kinase (CDK) inhibitors; and by interacting with the suppressor of variegation 3-9 homolog family protein (SUV39H1) to mediate H3K methylation, thereby silencing transcriptional activity of target genes. MEN1-associated tumors harbor germline and somatic mutations, consistent with Knudson's two-hit hypothesis. Genetic diagnosis to identify individuals with germline MEN1 mutations has facilitated appropriate targeting of clinical, biochemical and radiological screening for this high risk group of patients for whom earlier implementation of treatments can then be considered. MEN4 is caused by heterozygous mutations of CDNK1B which encodes the 196 amino-acid CDK1 p27Kip1, which is activated by H3K4 methylation.
Keywords: Histone methylation; Neuroendocrine tumors; Pancreatic islet; Parathyroid; Pituitary; Tumors.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures



References
-
- Agarwal S.K., Kester M.B., Debelenko L.V., Heppner C., Emmert-Buck M.R., Skarulis M.C. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum. Mol. Genet. 1997;6:1169–1175. - PubMed
-
- Agarwal S.K., Guru S.C., Heppner C., Erdos M.R., Collins R.M., Park S.Y. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–152. - PubMed
-
- Akerstrom G., Hellman P. Surgery on neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:87–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

