Tianeptine interferes with microtubule organization and hormone secretion of pheochromocytoma cells
- PMID: 23933152
- PMCID: PMC3796025
- DOI: 10.1016/j.mce.2013.07.033
Tianeptine interferes with microtubule organization and hormone secretion of pheochromocytoma cells
Abstract
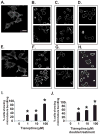
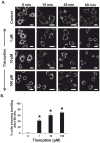
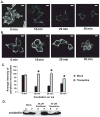
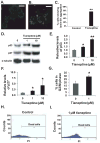
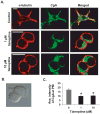
Pheochromocytoma originates from chromaffin cells in the adrenal medulla and sympathetic paraganglia. 36-53% of pheochromocytoma becomes malignant and, thereafter, resistant to conventional treatments. Pheochromocytoma also causes hyper-secretion of catecholamines that cause severe hypertension. We found that an antidepressant, tianeptine, interfered with normal life cycle of pheochromocytoma cells at its clinical doses. Treatment with tianeptine caused microtubule bundling and specific degradation of cytoplasmic dynein, a retrograde microtubule motor that mediates various microtubule-dependent processes during interphase and mitosis, in the rat pheochromocytoma PC12 cells. Tianeptine also increased the levels of pro-apoptotic proteins, slowed cell cycle progression, and increased apoptosis in PC12 cells. Importantly, tianeptine treatment decreased high K(+)-stimulated secretion of norepinephrine and chromogranin A in PC12 cells and of epinephrine in the mouse pheochromocytoma MPC cells. Our study demonstrates, for the first time, that tianeptine interferes with normal life cycle of pheochromocytoma cells.
Keywords: Cytoplasmic dynein; Microtubule bundling; Pheochromocytoma; Tianeptine.
Published by Elsevier Ireland Ltd.
Figures



Similar articles
-
Polyoma-induced neoplasms of the mouse adrenal medulla. Characterization of the tumors and establishment of cell lines.Lab Invest. 1993 May;68(5):541-9. Lab Invest. 1993. PMID: 8098784
-
Malignant and benign pheochromocytoma: chromaffin granule transmitters and the response to medical and surgical treatment.Ann N Y Acad Sci. 2002 Oct;971:530-2. doi: 10.1111/j.1749-6632.2002.tb04519.x. Ann N Y Acad Sci. 2002. PMID: 12438175 Clinical Trial. No abstract available.
-
Cytoplasmic dynein mediates adenovirus binding to microtubules.J Virol. 2004 Sep;78(18):10122-32. doi: 10.1128/JVI.78.18.10122-10132.2004. J Virol. 2004. PMID: 15331745 Free PMC article.
-
[Dynein and dynactin as organizers of the system of cell microtubules].Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
-
Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review.
References
-
- Adler JT, Meyer-Rochow GY, Chen H, Benn DE, Robinson BG, Sippel RS, Sidhu SB. Pheochromocytoma: current approaches and future directions. Oncologist. 2008;13:779–93. - PubMed
-
- Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–64. - PubMed
-
- Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–8. - PubMed
-
- Aonuma M, Miyamoto M, Inoue YH, Tamai K, Sakai H, Kamasawa N, Matsukage A. Microtubule bundle formation and cell death induced by the human CLASP/Orbit N-terminal fragment. Cell Struct Funct. 2005;30:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

