Aging is not a disease: distinguishing age-related macular degeneration from aging
- PMID: 23933169
- PMCID: PMC3830684
- DOI: 10.1016/j.preteyeres.2013.07.003
Aging is not a disease: distinguishing age-related macular degeneration from aging
Abstract
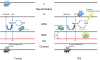
Age-related macular degeneration (AMD) is a disease of the outer retina, characterized most significantly by atrophy of photoreceptors and retinal pigment epithelium accompanied with or without choroidal neovascularization. Development of AMD has been recognized as contingent on environmental and genetic risk factors, the strongest being advanced age. In this review, we highlight pathogenic changes that destabilize ocular homeostasis and promote AMD development. With normal aging, photoreceptors are steadily lost, Bruch's membrane thickens, the choroid thins, and hard drusen may form in the periphery. In AMD, many of these changes are exacerbated in addition to the development of disease-specific factors such as soft macular drusen. Para-inflammation, which can be thought of as an intermediate between basal and robust levels of inflammation, develops within the retina in an attempt to maintain ocular homeostasis, reflected by increased expression of the anti-inflammatory cytokine IL-10 coupled with shifts in macrophage plasticity from the pro-inflammatory M1 to the anti-inflammatory M2 polarization. In AMD, imbalances in the M1 and M2 populations together with activation of retinal microglia are observed and potentially contribute to tissue degeneration. Nonetheless, the retina persists in a state of chronic inflammation and increased expression of certain cytokines and inflammasomes is observed. Since not everyone develops AMD, the vital question to ask is how the body establishes a balance between normal age-related changes and the pathological phenotypes in AMD.
Keywords: Age-related macular degeneration; Aging; Homeostasis; Oxidative stress; Para-inflammation; Retina.
Published by Elsevier Ltd.
Figures






References
-
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kahari VM. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22:2121–2134. - PubMed
-
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. - PubMed
-
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

