Depletion of extracellular signal-regulated kinase 1 in mice with cardiomyopathy caused by lamin A/C gene mutation partially prevents pathology before isoenzyme activation
- PMID: 23933734
- PMCID: PMC3857940
- DOI: 10.1093/hmg/ddt387
Depletion of extracellular signal-regulated kinase 1 in mice with cardiomyopathy caused by lamin A/C gene mutation partially prevents pathology before isoenzyme activation
Abstract
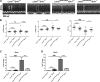
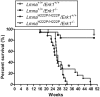
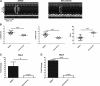
Mutations in the lamin A/C gene (LMNA) encoding A-type nuclear lamins cause dilated cardiomyopathy with variable muscular dystrophy. These mutations enhance mitogen-activated protein kinase signaling in the heart and pharmacological inhibition of extracellular signal-regulated kinase (ERK) 1 and 2 improves cardiac function in Lmna(H222P/H222P) mice. In the current study, we crossed mice lacking ERK1 to Lmna(H222P/H222P) mice and examined cardiac performance and survival. Male Lmna(H222P/H222P)/Erk1(-/-) mice lacking ERK1 had smaller left ventricular end systolic diameters and increased fractional shortening (FS) at 16 weeks of age than Lmna(H222P/H222P/)Erk1(+/+) mice. Their mean survival was also significantly longer. However, the improved cardiac function was abrogated at 20 weeks of age concurrent with an increased activity of ERK2. Lmna(H222P/H222P)/Erk1(-/-) mice treated with an inhibitor of ERK1/2 activation had smaller left ventricular diameters and increased FS at 20 weeks of age. These results provide genetic evidence that ERK1 and ERK2 contribute to the development of cardiomyopathy caused by LMNA mutations and reveal interplay between these isoenzymes in maintaining a combined pathological activity in heart.
Figures





References
-
- Dauer W.T., Worman H.J. The nuclear envelope as a signaling node in development and disease. Dev. Cell. 2009;17:626–638. - PubMed
-
- Bonne G., Di Barletta M.R., Varnous S., Bécane H.M., Hammouda E.H., Merlini L., Muntoni F., Greenberg C.R., Gary F., Urtizberea J.A., et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nat. Genet. 1999;21:285–288. - PubMed
-
- Fatkin D., MacRae C., Sasaki T., Wolff M.R., Porcu M., Frenneaux M., Atherton J., Vidaillet H.J., Spudich S., De Girolami U., et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999;341:1715–1724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

