Tuned thalamic excitation is amplified by visual cortical circuits
- PMID: 23933748
- PMCID: PMC3774518
- DOI: 10.1038/nn.3488
Tuned thalamic excitation is amplified by visual cortical circuits
Erratum in
-
Publisher Correction: Tuned thalamic excitation is amplified by visual cortical circuits.Nat Neurosci. 2018 Sep;21(9):1292. doi: 10.1038/s41593-018-0181-6. Nat Neurosci. 2018. PMID: 30013170
Abstract
Cortical neurons in thalamic recipient layers receive excitation from the thalamus and the cortex. The relative contribution of these two sources of excitation to sensory tuning is poorly understood. We optogenetically silenced the visual cortex of mice to isolate thalamic excitation onto layer 4 neurons during visual stimulation. Thalamic excitation contributed to a third of the total excitation and was organized in spatially offset, yet overlapping, ON and OFF receptive fields. This receptive field structure predicted the orientation tuning of thalamic excitation. Finally, both thalamic and total excitation were similarly tuned to orientation and direction and had the same temporal phase relationship to the visual stimulus. Our results indicate that tuning of thalamic excitation is unlikely to be imparted by direction- or orientation-selective thalamic neurons and that a principal role of cortical circuits is to amplify tuned thalamic excitation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

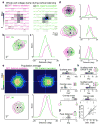


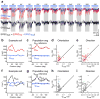


Comment in
-
A finely tuned cortical amplifier.Nat Neurosci. 2013 Sep;16(9):1166-8. doi: 10.1038/nn.3507. Nat Neurosci. 2013. PMID: 23982448 No abstract available.
References
-
- Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science. 1994;265:774–7. - PubMed
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. Journal of neurophysiology. 2000;84:909–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

