Inflammation-induced interstitial migration of effector CD4⁺ T cells is dependent on integrin αV
- PMID: 23933892
- PMCID: PMC4159184
- DOI: 10.1038/ni.2682
Inflammation-induced interstitial migration of effector CD4⁺ T cells is dependent on integrin αV
Abstract
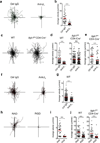
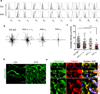
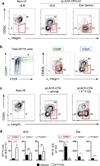
Leukocytes must traverse inflamed tissues to effectively control local infection. Although motility in dense tissues seems to be integrin independent and based on actomyosin-mediated protrusion and contraction, during inflammation, changes to the extracellular matrix (ECM) may necessitate distinct motility requirements. Indeed, we found that the interstitial motility of T cells was critically dependent on Arg-Gly-Asp (RGD)-binding integrins in the inflamed dermis. Inflammation-induced deposition of fibronectin was functionally linked to higher expression of integrin αV on effector CD4⁺ T cells. By intravital multiphoton imaging, we found that the motility of CD4⁺ T cells was dependent on αV expression. Selective blockade or knockdown of αV arrested T helper type 1 (TH1) cells in the inflamed tissue and attenuated local effector function. Our data demonstrate context-dependent specificity of lymphocyte movement in inflamed tissues that is essential for protective immunity.
Figures
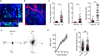



Comment in
-
Crawling of effector T cells on extracellular matrix: role of integrins in interstitial migration in inflamed tissues.Cell Mol Immunol. 2014 Jan;11(1):1-4. doi: 10.1038/cmi.2013.47. Epub 2013 Sep 23. Cell Mol Immunol. 2014. PMID: 24056781 Free PMC article. No abstract available.
References
-
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. - PubMed
-
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. - PubMed
-
- Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. - PubMed
-
- Woolf E, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI053746/AI/NIAID NIH HHS/United States
- R21 AI088427/AI/NIAID NIH HHS/United States
- HL087088/HL/NHLBI NIH HHS/United States
- P01 HL018208/HL/NHLBI NIH HHS/United States
- R01 AI072690/AI/NIAID NIH HHS/United States
- R37 AI072690/AI/NIAID NIH HHS/United States
- R01 HL087088/HL/NHLBI NIH HHS/United States
- AI072690/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- AI088427/AI/NIAID NIH HHS/United States
- R01 DK093695/DK/NIDDK NIH HHS/United States
- F32 AI089079/AI/NIAID NIH HHS/United States
- R01 AI070826/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

