Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer
- PMID: 23934154
- PMCID: PMC11113847
- DOI: 10.1007/s00018-013-1439-5
Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer
Abstract
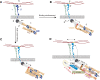
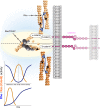
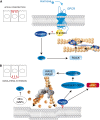
Mechanotransduction encompasses the role of mechanical forces in controlling cell behavior by activating signal transduction pathways. Most forces at a cellular level are caused by myosin II, which contracts and cross-links actin. Myosin II-dependent forces are transmitted through the actin cytoskeleton to molecular endpoints that promote specific cellular outcomes, e.g., cell proliferation, adhesion, or migration. For example, most adhesive and migratory phenomena are mechanically linked by a molecular clutch comprised of mechanosensitive scaffolds. Myosin II activation and mechanosensitive molecular mechanisms are finely tuned and spatiotemporally integrated to coordinate morphogenetic events during development. Mechanical events dependent on myosin II also participate in tumor cell proliferation, invasion, and metastatic dissemination. Specifically, tumor cells alter the mechanical properties of the microenvironment to create favorable conditions for proliferation and/or dissemination. These observations position myosin II-dependent force generation and mechanotransduction at the crossroads between normal development and cancer.
Figures

References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

