Tissue damage detection by osmotic surveillance
- PMID: 23934216
- PMCID: PMC3826879
- DOI: 10.1038/ncb2818
Tissue damage detection by osmotic surveillance
Abstract
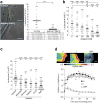
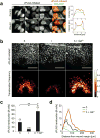
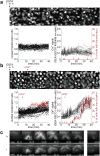
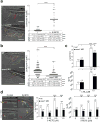
How tissue damage is detected to induce inflammatory responses is unclear. Most studies have focused on damage signals released by cell breakage and necrosis. Whether tissues use other cues in addition to cell lysis to detect that they are damaged is unknown. We find that osmolarity differences between interstitial fluid and the external environment mediate rapid leukocyte recruitment to sites of tissue damage in zebrafish by activating cytosolic phospholipase a2 (cPLA2) at injury sites. cPLA2 initiates the production of non-canonical arachidonate metabolites that mediate leukocyte chemotaxis through a 5-oxo-ETE receptor (OXE-R). Thus, tissues can detect damage through direct surveillance of barrier integrity, with cell swelling probably functioning as a pro-inflammatory intermediate in the process.
Figures

References
-
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. - PubMed
-
- Zhao M, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. - PubMed
-
- Gilljam H, Ellin A, Strandvik B. Increased bronchial chloride concentration in cystic fibrosis. Scand J Clin Lab Invest. 1989;49:121–124. - PubMed
-
- Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148:1633–1637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

