Fanconi anemia signaling network regulates the spindle assembly checkpoint
- PMID: 23934222
- PMCID: PMC3754252
- DOI: 10.1172/JCI67364
Fanconi anemia signaling network regulates the spindle assembly checkpoint
Abstract
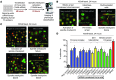
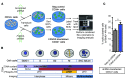
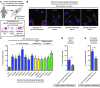
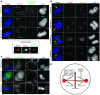
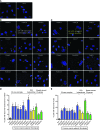
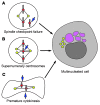
Fanconi anemia (FA) is a heterogenous genetic disease with a high risk of cancer. The FA proteins are essential for interphase DNA damage repair; however, it is incompletely understood why FA-deficient cells also develop gross aneuploidy, leading to cancer. Here, we systematically evaluated the role of the FA proteins in chromosome segregation through functional RNAi screens and analysis of primary cells from patients with FA. We found that FA signaling is essential for the spindle assembly checkpoint and is therefore required for high-fidelity chromosome segregation and prevention of aneuploidy. Furthermore, we discovered that FA proteins differentially localize to key structures of the mitotic apparatus in a cell cycle-dependent manner. The essential role of the FA pathway in mitosis offers a mechanistic explanation for the aneuploidy and malignant transformation known to occur after disruption of FA signaling. Collectively, our findings provide insight into the genetically unstable cancers resulting from inactivation of the FA/BRCA pathway.
Figures
References
-
- de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat Res. 2009;668(1–2):11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

