In vivo ectopic implantation model to assess human mesenchymal progenitor cell potential
- PMID: 23934266
- PMCID: PMC3834175
- DOI: 10.1007/s12015-013-9464-1
In vivo ectopic implantation model to assess human mesenchymal progenitor cell potential
Abstract
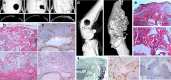
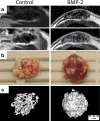
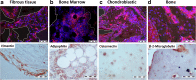
Clinical interest on human mesenchymal progenitor cells (hMPC) relies on their potential applicability in cell-based therapies. An in vitro characterization is usually performed in order to define MPC potency. However, in vitro predictions not always correlate with in vivo results and thus there is no consensus in how to really assess cell potency. Our goal was to provide an in vivo testing method to define cell behavior before therapeutic usage, especially for bone tissue engineering applications. In this context, we wondered whether bone marrow stromal cells (hBMSC) would proceed in an osteogenic microenvironment. Based on previous approaches, we developed a fibrin/ceramic/BMP-2/hBMSCs compound. We implanted the compound during only 2 weeks in NOD-SCID mice, either orthotopically to assess its osteoinductive property or subcutaneously to analyze its adequacy as a cell potency testing method. Using fluorescent cell labeling and immunohistochemistry techniques, we could ascertain cell differentiation to bone, bone marrow, cartilage, adipocyte and fibrous tissue. We observed differences in cell potential among different batches of hBMSCs, which did not strictly correlate with in vitro analyses. Our data indicate that the method we have developed is reliable, rapid and reproducible to define cell potency, and may be useful for testing cells destined to bone tissue engineering purposes. Additionally, results obtained with hMPCs from other sources indicate that our method is suitable for testing any potentially implantable mesenchymal cell. Finally, we propose that this model could successfully be employed for bone marrow niche and bone tumor studies.
Figures



References
-
- Backly, R., & Cancedda, R. (2010). Bone marrow stem cells in clinical application: harnessing paracrine roles and niche mechanisms. Bioreactor Systems for Tissue Engineering II, 265–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

