Nitrite reductase NirBD is induced and plays an important role during in vitro dormancy of Mycobacterium tuberculosis
- PMID: 23935045
- PMCID: PMC3807446
- DOI: 10.1128/JB.00698-13
Nitrite reductase NirBD is induced and plays an important role during in vitro dormancy of Mycobacterium tuberculosis
Abstract
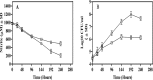
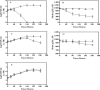
Mycobacterium tuberculosis is one of the strongest reducers of nitrate among all mycobacteria. Reduction of nitrate to nitrite, mediated by nitrate reductase (NarGHJI) of M. tuberculosis, is induced during the dormant stage, and the enzyme has a respiratory function in the absence of oxygen. Nitrite reductase (NirBD) is also functional during aerobic growth when nitrite is the sole nitrogen source. However, the role of NirBD-mediated nitrite reduction during the dormancy is not yet characterized. Here, we analyzed nitrite reduction during aerobic growth as well as in a hypoxic dormancy model of M. tuberculosis in vitro. When nitrite was used as the sole nitrogen source in the medium, the organism grew and the reduction of nitrite was evident in both hypoxic and aerobic cultures of M. tuberculosis. Remarkably, the hypoxic culture of M. tuberculosis, compared to the aerobic culture, showed 32- and 4-fold-increased expression of nitrite reductase (NirBD) at the transcription and protein levels, respectively. More importantly, a nirBD mutant of M. tuberculosis was unable to reduce nitrite and compared to the wild-type (WT) strain had a >2-log reduction in viability after 240 h in the Wayne model of hypoxic dormancy. Dependence of M. tuberculosis on nitrite reductase (NirBD) was also seen in a human macrophage-based dormancy model where the nirBD mutant was impaired for survival compared to the WT strain. Overall, the increased expression and essentiality of nitrite reductase in the in vitro dormancy models suggested that NirBD-mediated nitrite reduction could be critical during the persistent stage of M. tuberculosis.
Figures


References
-
- WHO 2012. Tuberculosis. Fact sheet no. 104 WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs104/en/index.html.
-
- Tufariello JM, Chan J, Flynn JL. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 3:578–590 - PubMed
-
- Cosma CL, Sherman DR, Ramakrishnan L. 2003. The secret lives of the pathogenic mycobacteria. Annu. Rev. Microbiol. 57:641–676 - PubMed
-
- Gomez JE, McKinney JD. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 84:29–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

