Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation
- PMID: 23935250
- PMCID: PMC3713603
- DOI: 10.1155/2013/893521
Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation
Abstract
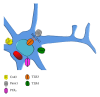
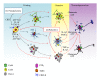
In the central nervous system (CNS), mastocytes and glial cells (microglia, astrocytes and oligodendrocytes) function as sensors of neuroinflammatory conditions, responding to stress triggers or becoming sensitized to subsequent proinflammatory challenges. The corticotropin-releasing hormone and glucocorticoids are critical players in stress-induced mastocyte degranulation and potentiation of glial inflammatory responses, respectively. Mastocytes and glial cells express different toll-like receptor (TLR) family members, and their activation via proinflammatory molecules can increase the expression of connexin hemichannels and pannexin channels in glial cells. These membrane pores are oligohexamers of the corresponding protein subunits located in the cell surface. They allow ATP release and Ca(2+) influx, which are two important elements of inflammation. Consequently, activated microglia and astrocytes release ATP and glutamate, affecting myelinization, neuronal development, and survival. Binding of ligands to TLRs induces a cascade of intracellular events leading to activation of several transcription factors that regulate the expression of many genes involved in inflammation. During pregnancy, the previous responses promoted by viral infections and other proinflammatory conditions are common and might predispose the offspring to develop psychiatric disorders and neurological diseases. Such disorders could eventually be potentiated by stress and might be part of the etiopathogenesis of CNS dysfunctions including autism spectrum disorders and schizophrenia.
Figures
References
-
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sciences. 1990;46(9):607–617. - PubMed
-
- Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Molecular Neurobiology. 2006;34(3):221–242. - PubMed
-
- Rivest S. Regulation of innate immune responses in the brain. Nature Reviews Immunology. 2009;9(6):429–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

