A multi-targeted drug candidate with dual anti-HIV and anti-HSV activity
- PMID: 23935482
- PMCID: PMC3723632
- DOI: 10.1371/journal.ppat.1003456
A multi-targeted drug candidate with dual anti-HIV and anti-HSV activity
Abstract
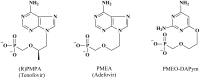
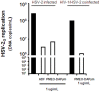
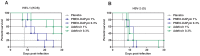
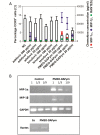
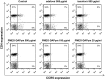
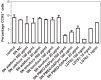
Human immunodeficiency virus (HIV) infection is often accompanied by infection with other pathogens, in particular herpes simplex virus type 2 (HSV-2). The resulting coinfection is involved in a vicious circle of mutual facilitations. Therefore, an important task is to develop a compound that is highly potent against both viruses to suppress their transmission and replication. Here, we report on the discovery of such a compound, designated PMEO-DAPym. We compared its properties with those of the structurally related and clinically used acyclic nucleoside phosphonates (ANPs) tenofovir and adefovir. We demonstrated the potent anti-HIV and -HSV activity of this drug in a diverse set of clinically relevant in vitro, ex vivo, and in vivo systems including (i) CD4⁺ T-lymphocyte (CEM) cell cultures, (ii) embryonic lung (HEL) cell cultures, (iii) organotypic epithelial raft cultures of primary human keratinocytes (PHKs), (iv) primary human monocyte/macrophage (M/M) cell cultures, (v) human ex vivo lymphoid tissue, and (vi) athymic nude mice. Upon conversion to its diphosphate metabolite, PMEO-DAPym markedly inhibits both HIV-1 reverse transcriptase (RT) and HSV DNA polymerase. However, in striking contrast to tenofovir and adefovir, it also acts as an efficient immunomodulator, inducing β-chemokines in PBMC cultures, in particular the CCR5 agonists MIP-1β, MIP-1α and RANTES but not the CXCR4 agonist SDF-1, without the need to be intracellularly metabolized. Such specific β-chemokine upregulation required new mRNA synthesis. The upregulation of β-chemokines was shown to be associated with a pronounced downmodulation of the HIV-1 coreceptor CCR5 which may result in prevention of HIV entry. PMEO-DAPym belongs conceptually to a new class of efficient multitargeted antivirals for concomitant dual-viral (HSV/HIV) infection therapy through inhibition of virus-specific pathways (i.e. the viral polymerases) and HIV transmission prevention through interference with host pathways (i.e. CCR5 receptor down regulation).
Conflict of interest statement
Jan Balzarini and Antonin Holý are co-inventors of the acyclic nucleoside phosphonates. This did not alter our adherence to all PLOS Pathogens policies on sharing data and materials. The other authors declare no competing financial interests.
Figures





References
-
- Blower S, Ma L (2004) Calculating the contribution of herpes simplex virus type 2 epidemics to increasing HIV incidence: treatment implications. Clin Infect Dis 39, Suppl 5: S240–S247. - PubMed
-
- Corey L (2007) Synergistic copathogens–HIV-1 and HSV-2. N Engl J Med 356: 854–856. - PubMed
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, et al. (2006) Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20: 73–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

