Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets
- PMID: 23935497
- PMCID: PMC3731246
- DOI: 10.1371/journal.ppat.1003513
Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets
Abstract
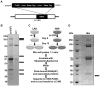
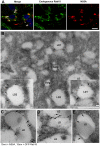
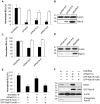
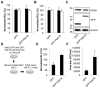
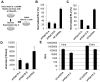
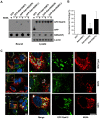
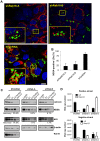
Hepatitis C virus (HCV) is a single-stranded RNA virus that replicates on endoplasmic reticulum-derived membranes. HCV particle assembly is dependent on the association of core protein with cellular lipid droplets (LDs). However, it remains uncertain whether HCV assembly occurs at the LD membrane itself or at closely associated ER membranes. Furthermore, it is not known how the HCV replication complex and progeny genomes physically associate with the presumed sites of virion assembly at or near LDs. Using an unbiased proteomic strategy, we have found that Rab18 interacts with the HCV nonstructural protein NS5A. Rab18 associates with LDs and is believed to promote physical interaction between LDs and ER membranes. Active (GTP-bound) forms of Rab18 bind more strongly to NS5A than a constitutively GDP-bound mutant. NS5A colocalizes with Rab18-positive LDs in HCV-infected cells, and Rab18 appears to promote the physical association of NS5A and other replicase components with LDs. Modulation of Rab18 affects genome replication and possibly also the production of infectious virions. Our results support a model in which specific interactions between viral and cellular proteins may promote the physical interaction between membranous HCV replication foci and lipid droplets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Brass V, Bieck E, Montserret R, Wölk B, Hellings JA, et al. (2002) An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J Biol Chem 277: 8130–8139. - PubMed
-
- Penin F, Brass V, Appel N, Ramboarina S, Montserret R, et al. (2004) Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J Biol Chem 279: 40835–40843. - PubMed
-
- Tellinghuisen TL, Marcotrigiano J, Gorbalenya AE, Rice CM (2004) The NS5A protein of hepatitis C virus is a zinc metalloprotein. J Biol Chem 279: 48576–48587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

