A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes
- PMID: 23935504
- PMCID: PMC3731245
- DOI: 10.1371/journal.ppat.1003529
A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes
Abstract
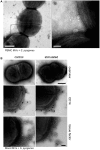
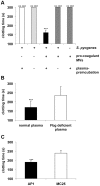
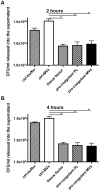
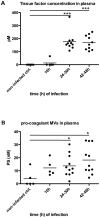
Previous studies have shown that stimulation of whole blood or peripheral blood mononuclear cells with bacterial virulence factors results in the sequestration of pro-coagulant microvesicles (MVs). These particles explore their clotting activity via the extrinsic and intrinsic pathway of coagulation; however, their pathophysiological role in infectious diseases remains enigmatic. Here we describe that the interaction of pro-coagulant MVs with bacteria of the species Streptococcus pyogenes is part of the early immune response to the invading pathogen. As shown by negative staining electron microscopy and clotting assays, pro-coagulant MVs bind in the presence of plasma to the bacterial surface. Fibrinogen was identified as a linker that, through binding to the M1 protein of S. pyogenes, allows the opsonization of the bacteria by MVs. Surface plasmon resonance analysis revealed a strong interaction between pro-coagulant MVs and fibrinogen with a KD value in the nanomolar range. When performing a mass-spectrometry-based strategy to determine the protein quantity, a significant up-regulation of the fibrinogen-binding integrins CD18 and CD11b on pro-coagulant MVs was recorded. Finally we show that plasma clots induced by pro-coagulant MVs are able to prevent bacterial dissemination and possess antimicrobial activity. These findings were confirmed by in vivo experiments, as local treatment with pro-coagulant MVs dampens bacterial spreading to other organs and improved survival in an invasive streptococcal mouse model of infection. Taken together, our data implicate that pro-coagulant MVs play an important role in the early response of the innate immune system in infectious diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The role of coagulation/fibrinolysis during Streptococcus pyogenes infection.Front Cell Infect Microbiol. 2014 Sep 11;4:128. doi: 10.3389/fcimb.2014.00128. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25309880 Free PMC article. Review.
-
Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and pro-inflammatory microparticles.Cell Microbiol. 2012 Jan;14(1):107-19. doi: 10.1111/j.1462-5822.2011.01705.x. Epub 2011 Nov 10. Cell Microbiol. 2012. PMID: 21951918
-
Local activation of coagulation factor XIII reduces systemic complications and improves the survival of mice after Streptococcus pyogenes M1 skin infection.Int J Med Microbiol. 2016 Nov;306(7):572-579. doi: 10.1016/j.ijmm.2016.06.001. Epub 2016 Jun 8. Int J Med Microbiol. 2016. PMID: 27338836
-
M protein from Streptococcus pyogenes induces tissue factor expression and pro-coagulant activity in human monocytes.Microbiology (Reading). 2007 Aug;153(Pt 8):2458-2464. doi: 10.1099/mic.0.2006/003285-0. Microbiology (Reading). 2007. PMID: 17660410 Free PMC article.
-
Proteolysis and its regulation at the surface of Streptococcus pyogenes.Mol Microbiol. 2002 Feb;43(3):537-44. doi: 10.1046/j.1365-2958.2002.02766.x. Mol Microbiol. 2002. PMID: 11929513 Review.
Cited by
-
Exosome: A New Player in Translational Nanomedicine.J Clin Med. 2020 Jul 26;9(8):2380. doi: 10.3390/jcm9082380. J Clin Med. 2020. PMID: 32722531 Free PMC article. Review.
-
The role of coagulation/fibrinolysis during Streptococcus pyogenes infection.Front Cell Infect Microbiol. 2014 Sep 11;4:128. doi: 10.3389/fcimb.2014.00128. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25309880 Free PMC article. Review.
-
Interaction of the Human Contact System with Pathogens-An Update.Front Immunol. 2018 Feb 26;9:312. doi: 10.3389/fimmu.2018.00312. eCollection 2018. Front Immunol. 2018. PMID: 29535715 Free PMC article. Review.
-
Non-coding RNAs and Exosomes: Their Role in the Pathogenesis of Sepsis.Mol Ther Nucleic Acids. 2020 Sep 4;21:51-74. doi: 10.1016/j.omtn.2020.05.012. Epub 2020 May 15. Mol Ther Nucleic Acids. 2020. PMID: 32506014 Free PMC article. Review.
-
The contact system proteases play disparate roles in streptococcal sepsis.Haematologica. 2020 May;105(5):1424-1435. doi: 10.3324/haematol.2019.223545. Epub 2019 Jul 18. Haematologica. 2020. PMID: 31320552 Free PMC article.
References
-
- Esmon CT, Xu J, Lupu F (2011) Innate immunity and coagulation. J Thromb Haemost 9 Suppl 1: 182–188 doi:10.1111/j.1538-7836.2011.04323.x - DOI - PMC - PubMed
-
- Hanington PC, Zhang S-M (2011) The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J Innate Immun 3: 17–27 doi:10.1159/000321882 - DOI - PMC - PubMed
-
- Krem MM, Di Cera E (2002) Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci 27: 67–74. - PubMed
-
- Loof TG, Schmidt O, Herwald H, Theopold U (2011) Coagulation Systems of Invertebrates and Vertebrates and Their Roles in Innate Immunity: The Same Side of Two Coins? J Innate Immun 3: 34–40. - PubMed
-
- Furie B, Furie BC (1988) The molecular basis of blood coagulation. Cell 53: 505–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

