A network of HMG-box transcription factors regulates sexual cycle in the fungus Podospora anserina
- PMID: 23935511
- PMCID: PMC3730723
- DOI: 10.1371/journal.pgen.1003642
A network of HMG-box transcription factors regulates sexual cycle in the fungus Podospora anserina
Abstract
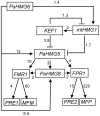
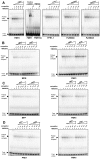
High-mobility group (HMG) B proteins are eukaryotic DNA-binding proteins characterized by the HMG-box functional motif. These transcription factors play a pivotal role in global genomic functions and in the control of genes involved in specific developmental or metabolic pathways. The filamentous ascomycete Podospora anserina contains 12 HMG-box genes. Of these, four have been previously characterized; three are mating-type genes that control fertilization and development of the fruit-body, whereas the last one encodes a factor involved in mitochondrial DNA stability. Systematic deletion analysis of the eight remaining uncharacterized HMG-box genes indicated that none were essential for viability, but that seven were involved in the sexual cycle. Two HMG-box genes display striking features. PaHMG5, an ortholog of SpSte11 from Schizosaccharomyces pombe, is a pivotal activator of mating-type genes in P. anserina, whereas PaHMG9 is a repressor of several phenomena specific to the stationary phase, most notably hyphal anastomoses. Transcriptional analyses of HMG-box genes in HMG-box deletion strains indicated that PaHMG5 is at the hub of a network of several HMG-box factors that regulate mating-type genes and mating-type target genes. Genetic analyses revealed that this network also controls fertility genes that are not regulated by mating-type transcription factors. This study points to the critical role of HMG-box members in sexual reproduction in fungi, as 11 out of 12 members were involved in the sexual cycle in P. anserina. PaHMG5 and SpSte11 are conserved transcriptional regulators of mating-type genes, although P. anserina and S. pombe diverged 550 million years ago. Two HMG-box genes, SOX9 and its upstream regulator SRY, also play an important role in sex determination in mammals. The P. anserina and S. pombe mating-type genes and their upstream regulatory factor form a module of HMG-box genes analogous to the SRY/SOX9 module, revealing a commonality of sex regulation in animals and fungi.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Bustin M (2001) Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci 26: 152–153. - PubMed
-
- Koopman, P. 2010. HMG Domain Superfamily of DNA-bending Proteins: HMG, UBF, TCF, LEF, SOX, SRY and Related Proteins. eLS. DOI: 10.1002/9780470015902.a0002325.pub2
-
- Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220: 35–46. - PubMed
-
- Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, et al. (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462: 99–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

