A dual role for SOX10 in the maintenance of the postnatal melanocyte lineage and the differentiation of melanocyte stem cell progenitors
- PMID: 23935512
- PMCID: PMC3723529
- DOI: 10.1371/journal.pgen.1003644
A dual role for SOX10 in the maintenance of the postnatal melanocyte lineage and the differentiation of melanocyte stem cell progenitors
Abstract
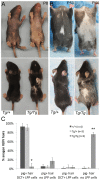
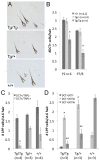
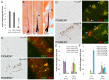
During embryogenesis, the transcription factor, Sox10, drives the survival and differentiation of the melanocyte lineage. However, the role that Sox10 plays in postnatal melanocytes is not established. We show in vivo that melanocyte stem cells (McSCs) and more differentiated melanocytes express SOX10 but that McSCs remain undifferentiated. Sox10 knockout (Sox10(fl); Tg(Tyr::CreER)) results in loss of both McSCs and differentiated melanocytes, while overexpression of Sox10 (Tg(DctSox10)) causes premature differentiation and loss of McSCs, leading to hair graying. This suggests that levels of SOX10 are key to normal McSC function and Sox10 must be downregulated for McSC establishment and maintenance. We examined whether the mechanism of Tg(DctSox10) hair graying is through increased expression of Mitf, a target of SOX10, by asking if haploinsufficiency for Mitf (Mitf(vga9) ) can rescue hair graying in Tg(DctSox10) animals. Surprisingly, Mitf(vga9) does not mitigate but exacerbates Tg(DctSox10) hair graying suggesting that MITF participates in the negative regulation of Sox10 in McSCs. These observations demonstrate that while SOX10 is necessary to maintain the postnatal melanocyte lineage it is simultaneously prevented from driving differentiation in the McSCs. This data illustrates how tissue-specific stem cells can arise from lineage-specified precursors through the regulation of the very transcription factors important in defining that lineage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Tobin DJ, Paus R (2001) Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol 36: 29–54. - PubMed
-
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, et al. (2002) Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416: 854–860 doi:10.1038/416854a - DOI - PubMed
-
- Mak S-S, Moriyama M, Nishioka E, Osawa M, Nishikawa S-I (2006) Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev Biol 291: 144–153 doi:10.1016/j.ydbio.2005.12.025 - DOI - PubMed
-
- Osawa M, Egawa G, Mak S-S, Moriyama M, Freter R, et al. (2005) Molecular characterization of melanocyte stem cells in their niche. Development 132: 5589–5599 doi:10.1242/dev.02161 - DOI - PubMed
-
- Botchkareva NV, Botchkarev VA, Gilchrest BA (2003) Fate of melanocytes during development of the hair follicle pigmentary unit. J Investig Dermatol Symp Proc 8: 76–79 doi:10.1046/j.1523-1747.2003.12176.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

