Loss of a conserved tRNA anticodon modification perturbs cellular signaling
- PMID: 23935536
- PMCID: PMC3731203
- DOI: 10.1371/journal.pgen.1003675
Loss of a conserved tRNA anticodon modification perturbs cellular signaling
Abstract
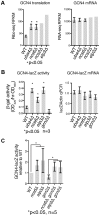
Transfer RNA (tRNA) modifications enhance the efficiency, specificity and fidelity of translation in all organisms. The anticodon modification mcm(5)s(2)U(34) is required for normal growth and stress resistance in yeast; mutants lacking this modification have numerous phenotypes. Mutations in the homologous human genes are linked to neurological disease. The yeast phenotypes can be ameliorated by overexpression of specific tRNAs, suggesting that the modifications are necessary for efficient translation of specific codons. We determined the in vivo ribosome distributions at single codon resolution in yeast strains lacking mcm(5)s(2)U. We found accumulations at AAA, CAA, and GAA codons, suggesting that translation is slow when these codons are in the ribosomal A site, but these changes appeared too small to affect protein output. Instead, we observed activation of the GCN4-mediated stress response by a non-canonical pathway. Thus, loss of mcm(5)s(2)U causes global effects on gene expression due to perturbation of cellular signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Agris PF, Vendeix FAP, Graham WD (2007) tRNA's wobble decoding of the genome: 40 years of modification. Journal of Molecular Biology 366: 1–13 doi:10.1016/j.jmb.2006.11.046 - DOI - PubMed
-
- Phizicky EM, Hopper AK (2010) tRNA biology charges to the front. Genes & Development 24: 1832–1860 doi:10.1101/gad.1956510 - DOI - PMC - PubMed
-
- Johansson M, Byström A (2005) Transfer RNA modifications and modifying enzymes in Saccharomyces cerevisiae. Fine-tuning of RNA functions by modification and editing 12: 87–120 doi:10.1007/b105814 - DOI
-
- Huang B, Johansson MJO, Byström AS (2005) An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11: 424–436 doi:10.1261/rna.7247705 - DOI - PMC - PubMed
-
- Leidel S, Pedrioli PGA, Bucher T, Brost R, Costanzo M, et al. (2009) Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 458: 228–232 doi:10.1038/nature07643 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

