Neuroanatomical differences in visual, motor, and language cortices between congenitally deaf signers, hearing signers, and hearing non-signers
- PMID: 23935567
- PMCID: PMC3731534
- DOI: 10.3389/fnana.2013.00026
Neuroanatomical differences in visual, motor, and language cortices between congenitally deaf signers, hearing signers, and hearing non-signers
Abstract
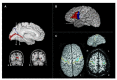
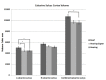
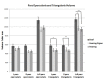
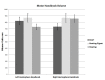
WE INVESTIGATED EFFECTS OF SIGN LANGUAGE USE AND AUDITORY DEPRIVATION FROM BIRTH ON THE VOLUMES OF THREE CORTICAL REGIONS OF THE HUMAN BRAIN: the visual cortex surrounding the calcarine sulcus in the occipital lobe; the language-related cortex in the inferior frontal gyrus (pars triangularis and pars opercularis); and the motor hand region in the precentral gyrus. The study included 25 congenitally deaf participants and 41 hearing participants (of which 16 were native sign language users); all were right-handed. Deaf participants exhibited a larger calcarine volume than hearing participants, which we interpret as the likely result of cross-modal compensation and/or dynamic interactions within sensory neural networks. Deaf participants also had increased volumes of the pars triangularis bilaterally compared to hearing signers and non-signers, which we interpret is related to the increased linguistic demands of speech processing and/or text reading for deaf individuals. Finally, although no statistically significant differences were found in the motor hand region for any of the groups, the deaf group was leftward asymmetric, the hearing signers essentially symmetric and the hearing non-signers were rightward asymmetric - results we interpret as the possible result of activity-dependent change due to life-long signing. The brain differences we observed in visual, motor, and language-related areas in adult deaf native signers provide evidence for the plasticity available for cognitive adaptation to varied environments during development.
Keywords: calcarine; deaf; inferior frontal gyrus; morphometry; motor hand region; sign language.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

