Comodulation of dopamine and serotonin on prefrontal cortical rhythms: a theoretical study
- PMID: 23935568
- PMCID: PMC3733011
- DOI: 10.3389/fnint.2013.00054
Comodulation of dopamine and serotonin on prefrontal cortical rhythms: a theoretical study
Abstract
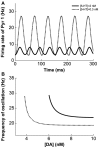
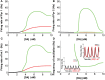
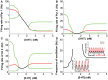
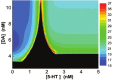
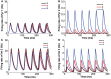
The prefrontal cortex (PFC) is implicated to play an important role in cognitive control. Abnormal PFC activities and rhythms have been observed in some neurological and neuropsychiatric disorders, and evidences suggest influences from the neuromodulators dopamine (DA) and serotonin (5-HT). Despite the high level of interest in these brain systems, the combined effects of DA and 5-HT modulation on PFC dynamics remain unknown. In this work, we build a mathematical model that incorporates available experimental findings to systematically study the comodulation of DA and 5-HT on the network behavior, focusing on beta and gamma band oscillations. Single neuronal model shows pyramidal cells with 5-HT1A and 2A receptors can be non-monotonically modulated by 5-HT. Two-population excitatory-inhibitory type network consisting of pyramidal cells with D1 receptors can provide rich repertoires of oscillatory behavior. In particular, 5-HT and DA can modulate the amplitude and frequency of the oscillations, which can emerge or cease, depending on receptor types. Certain receptor combinations are conducive for the robustness of the oscillatory regime, or the existence of multiple discrete oscillatory regimes. In a multi-population heterogeneous model that takes into account possible combination of receptors, we demonstrate that robust network oscillations require high DA concentration. We also show that selective D1 receptor antagonists (agonists) tend to suppress (enhance) network oscillations, increase the frequency from beta toward gamma band, while selective 5-HT1A antagonists (agonists) act in opposite ways. Selective D2 or 5-HT2A receptor antagonists (agonists) can lead to decrease (increase) in oscillation amplitude, but only 5-HT2A antagonists (agonists) can increase (decrease) the frequency. These results are comparable to some pharmacological effects. Our work illustrates the complex mechanisms of DA and 5-HT when operating simultaneously through multiple receptors.
Keywords: computational model; dopamine DA; nonlinear dynamics; prefrontal cortical circuit; selective dopamine and serotonin receptor agonist and antagonist; serotonin 5-HT.
Figures








References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

