New insights into the evolutionary history of biological nitrogen fixation
- PMID: 23935594
- PMCID: PMC3733012
- DOI: 10.3389/fmicb.2013.00201
New insights into the evolutionary history of biological nitrogen fixation
Abstract
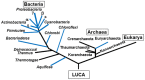
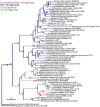
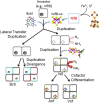
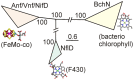
Nitrogenase, which catalyzes the ATP-dependent reduction of dinitrogen (N2) to ammonia (NH3), accounts for roughly half of the bioavailable nitrogen supporting extant life. The fundamental requirement for fixed forms of nitrogen for life on Earth, both at present and in the past, has led to broad and significant interest in the origin and evolution of biological N2 fixation. One key question is whether the limited availability of fixed nitrogen was a factor in life's origin or whether there were ample sources of fixed nitrogen produced by abiotic processes or delivered through the weathering of bolide impact materials to support this early life. If the latter, the key questions become what were the characteristics of the environment that precipitated the evolution of this oxygen sensitive process, when did this occur, and how was its subsequent evolutionary history impacted by the advent of oxygenic photosynthesis and the rise of oxygen in the Earth's biosphere. Since the availability of fixed sources of nitrogen capable of supporting early life is difficult to glean from the geologic record, there are limited means to get direct insights into these questions. Indirect insights, however, can be gained through phylogenetic studies of nitrogenase structural gene products and additional gene products involved in the biosynthesis of the complex metal-containing prosthetic groups associated with this enzyme complex. Insights gained from such studies, as reviewed herein, challenge traditional models for the evolution of biological nitrogen fixation and provide the basis for the development of new conceptual models that explain the stepwise evolution of this highly complex life sustaining process.
Keywords: NIf; great oxidation event; methanogens; nitrogen fixation.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

