NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells
- PMID: 23935876
- PMCID: PMC3728367
- DOI: 10.1371/journal.pone.0068597
NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells
Abstract
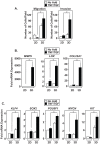
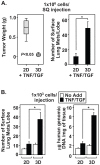
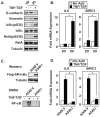
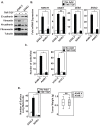
The epithelial-to-mesenchymal transition (EMT) is a de-differentiation process that has been implicated in metastasis and the generation of cancer initiating cells (CICs) in solid tumors. To examine EMT in non-small cell lung cancer (NSCLC), we utilized a three dimensional (3D) cell culture system in which cells were co-stimulated with tumor necrosis factor alpha (TNF) and transforming growth factor beta (TGFβ). NSCLC spheroid cultures display elevated expression of EMT master-switch transcription factors, TWIST1, SNAI1/Snail1, SNAI2/Slug and ZEB2/Sip1, and are highly invasive. Mesenchymal NSCLC cultures show CIC characteristics, displaying elevated expression of transcription factors KLF4, SOX2, POU5F1/Oct4, MYCN, and KIT. As a result, these putative CIC display a cancer "stem-like" phenotype by forming lung metastases under limiting cell dilution. The pleiotropic transcription factor, NF-κB, has been implicated in EMT and metastasis. Thus, we set out to develop a NSCLC model to further characterize the role of NF-κB activation in the development of CICs. Here, we demonstrate that induction of EMT in 3D cultures results in constitutive NF-κB activity. Furthermore, inhibition of NF-κB resulted in the loss of TWIST1, SNAI2, and ZEB2 induction, and a failure of cells to invade and metastasize. Our work indicates that NF-κB is required for NSCLC metastasis, in part, by transcriptionally upregulating master-switch transcription factors required for EMT.
Conflict of interest statement
Figures


References
-
- Cirri P, Chiarugi P (2012) Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev 31: 195–208 10.1007/s10555-011-9340-x - DOI - PubMed
-
- Korkaya H, Liu S, Wicha MS (2011) Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 121: 3804–3809 10.1172/JCI57099 - DOI - PMC - PubMed
-
- Fuxe J, Karlsson MC (2012) TGF-beta-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin Cancer Biol 22: 455–461 10.1016/j.semcancer.2012.05.004 - DOI - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890. - PubMed
-
- Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell 14: 818–829 10.1016/j.devcel.2008.05.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

