Effects of thyroxine exposure on osteogenesis in mouse calvarial pre-osteoblasts
- PMID: 23935926
- PMCID: PMC3720861
- DOI: 10.1371/journal.pone.0069067
Effects of thyroxine exposure on osteogenesis in mouse calvarial pre-osteoblasts
Abstract
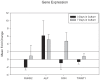
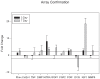
The incidence of craniosynostosis is one in every 1,800-2500 births. The gene-environment model proposes that if a genetic predisposition is coupled with environmental exposures, the effects can be multiplicative resulting in severely abnormal phenotypes. At present, very little is known about the role of gene-environment interactions in modulating craniosynostosis phenotypes, but prior evidence suggests a role for endocrine factors. Here we provide a report of the effects of thyroid hormone exposure on murine calvaria cells. Murine derived calvaria cells were exposed to critical doses of pharmaceutical thyroxine and analyzed after 3 and 7 days of treatment. Endpoint assays were designed to determine the effects of the hormone exposure on markers of osteogenesis and included, proliferation assay, quantitative ALP activity assay, targeted qPCR for mRNA expression of Runx2, Alp, Ocn, and Twist1, genechip array for 28,853 targets, and targeted osteogenic microarray with qPCR confirmations. Exposure to thyroxine stimulated the cells to express ALP in a dose dependent manner. There were no patterns of difference observed for proliferation. Targeted RNA expression data confirmed expression increases for Alp and Ocn at 7 days in culture. The genechip array suggests substantive expression differences for 46 gene targets and the targeted osteogenesis microarray indicated 23 targets with substantive differences. 11 gene targets were chosen for qPCR confirmation because of their known association with bone or craniosynostosis (Col2a1, Dmp1, Fgf1, 2, Igf1, Mmp9, Phex, Tnf, Htra1, Por, and Dcn). We confirmed substantive increases in mRNA for Phex, FGF1, 2, Tnf, Dmp1, Htra1, Por, Igf1 and Mmp9, and substantive decreases for Dcn. It appears thyroid hormone may exert its effects through increasing osteogenesis. Targets isolated suggest a possible interaction for those gene products associated with calvarial suture growth and homeostasis as well as craniosynostosis.
Conflict of interest statement
Figures


References
-
- Radetti G, Zavallone A, Gentili L, Beck-Peccoz P, Bona G (2002) Foetal and neonatal thyroid disorders. Minerva pediatrica 54: 383–400. - PubMed
-
- Menking M, Wiebel J, Schmid WU, Schmidt WT, Ebel KD, et al. (1972) Premature craniosynostosis associated with hyperthyroidism in 4 children with reference to 5 further cases in the literature. Monatsschrift fur Kinderheilkunde 120: 106–110. - PubMed
-
- Rasmussen SA, Yazdy MM, Carmichael SL, Jamieson DJ, Canfield MA, et al. (2007) Maternal thyroid disease as a risk factor for craniosynostosis. Obstetrics and gynecology 110: 369–377. - PubMed
-
- Riggs W Jr, Wilroy RS Jr, Etteldorf JN (1972) Neonatal hyperthyroidism with accelerated skeletal maturation, craniosynostosis, and brachydactyly. Radiology 105: 621–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

