Action of shiga toxin type-2 and subtilase cytotoxin on human microvascular endothelial cells
- PMID: 23936204
- PMCID: PMC3728274
- DOI: 10.1371/journal.pone.0070431
Action of shiga toxin type-2 and subtilase cytotoxin on human microvascular endothelial cells
Abstract
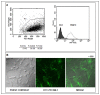
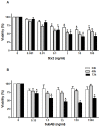
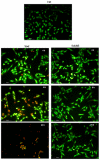
The hemolytic uremic syndrome (HUS) associated with diarrhea is a complication of Shiga toxin (Stx)-producing Escherichia coli (STEC) infection. In Argentina, HUS is endemic and responsible for acute and chronic renal failure in children younger than 5 years old. The human kidney is the most affected organ due to the presence of very Stx-sensitive cells, such as microvascular endothelial cells. Recently, Subtilase cytotoxin (SubAB) was proposed as a new toxin that may contribute to HUS pathogenesis, although its action on human glomerular endothelial cells (HGEC) has not been described yet. In this study, we compared the effects of SubAB with those caused by Stx2 on primary cultures of HGEC isolated from fragments of human pediatric renal cortex. HGEC were characterized as endothelial since they expressed von Willebrand factor (VWF) and platelet/endothelial cell adhesion molecule 1 (PECAM-1). HGEC also expressed the globotriaosylceramide (Gb3) receptor for Stx2. Both, Stx2 and SubAB induced swelling and detachment of HGEC and the consequent decrease in cell viability in a time-dependent manner. Preincubation of HGEC with C-9 -a competitive inhibitor of Gb3 synthesis-protected HGEC from Stx2 but not from SubAB cytotoxic effects. Stx2 increased apoptosis in a time-dependent manner while SubAB increased apoptosis at 4 and 6 h but decreased at 24 h. The apoptosis induced by SubAB relative to Stx2 was higher at 4 and 6 h, but lower at 24 h. Furthermore, necrosis caused by Stx2 was significantly higher than that induced by SubAB at all the time points evaluated. Our data provide evidence for the first time how SubAB could cooperate with the development of endothelial damage characteristic of HUS pathogenesis.
Conflict of interest statement
Figures



References
-
- Karpman D (2002) Haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Curr Paediatr 12.
-
- Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS et al. (1985) The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis 151: 775-782. doi:10.1093/infdis/151.5.775. PubMed: 3886804. - DOI - PubMed
-
- Repetto HA (1997) Epidemic hemolytic-uremic syndrome in children. Kidney Int 52: 1708-1719. doi:10.1038/ki.1997.508. PubMed: 9407523. - DOI - PubMed
-
- Ha Repetto, Arrizurieta E, Rivas M, Ibarra C (2009) Microangiopatía trombótica y Sindrome Hemolítico Urémico. Nefrología Clínica 3ra edición: 286-297
-
- Rivas M, Miliwebsky E, Chinen I, Deza N, Leotta GA (2006) The epidemiology of hemolytic uremic syndrome in Argentina. Diagnosis of the etiologic agent, reservoirs and routes of transmission]. Medicina (B Aires) 66 Suppl 3: 27-32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

