Determinants for simultaneous binding of copper and platinum to human chaperone Atox1: hitchhiking not hijacking
- PMID: 23936210
- PMCID: PMC3728025
- DOI: 10.1371/journal.pone.0070473
Determinants for simultaneous binding of copper and platinum to human chaperone Atox1: hitchhiking not hijacking
Abstract
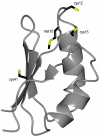
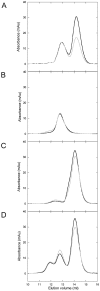
Cisplatin (CisPt) is an anticancer agent that has been used for decades to treat a variety of cancers. CisPt treatment causes many side effects due to interactions with proteins that detoxify the drug before reaching the DNA. One key player in CisPt resistance is the cellular copper-transport system involving the uptake protein Ctr1, the cytoplasmic chaperone Atox1 and the secretory path ATP7A/B proteins. CisPt has been shown to bind to ATP7B, resulting in vesicle sequestering of the drug. In addition, we and others showed that the apo-form of Atox1 could interact with CisPt in vitro and in vivo. Since the function of Atox1 is to transport copper (Cu) ions, it is important to assess how CisPt binding depends on Cu-loading of Atox1. Surprisingly, we recently found that CisPt interacted with Cu-loaded Atox1 in vitro at a position near the Cu site such that unique spectroscopic features appeared. Here, we identify the binding site for CisPt in the Cu-loaded form of Atox1 using strategic variants and a combination of spectroscopic and chromatographic methods. We directly prove that both metals can bind simultaneously and that the unique spectroscopic signals originate from an Atox1 monomer species. Both Cys in the Cu-site (Cys12, Cys15) are needed to form the di-metal complex, but not Cys41. Removing Met10 in the conserved metal-binding motif makes the loop more floppy and, despite metal binding, there are no metal-metal electronic transitions. In silico geometry minimizations provide an energetically favorable model of a tentative ternary Cu-Pt-Atox1 complex. Finally, we demonstrate that Atox1 can deliver CisPt to the fourth metal binding domain 4 of ATP7B (WD4), indicative of a possible drug detoxification mechanism.
Conflict of interest statement
Figures



Similar articles
-
Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6951-6. doi: 10.1073/pnas.1012899108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482801 Free PMC article.
-
Interaction between the anticancer drug Cisplatin and the copper chaperone Atox1 in human melanoma cells.Protein Pept Lett. 2014;21(1):63-8. doi: 10.2174/09298665113209990036. Protein Pept Lett. 2014. PMID: 23988033
-
T versus D in the MTCXXC motif of copper transport proteins plays a role in directional metal transport.J Biol Inorg Chem. 2014 Aug;19(6):1037-47. doi: 10.1007/s00775-014-1147-0. Epub 2014 May 14. J Biol Inorg Chem. 2014. PMID: 24824562
-
An expanding range of functions for the copper chaperone/antioxidant protein Atox1.Antioxid Redox Signal. 2013 Sep 20;19(9):945-57. doi: 10.1089/ars.2012.5086. Epub 2013 Feb 6. Antioxid Redox Signal. 2013. PMID: 23249252 Free PMC article. Review.
-
Extended functional repertoire for human copper chaperones.Biomol Concepts. 2016 Feb;7(1):29-39. doi: 10.1515/bmc-2015-0030. Biomol Concepts. 2016. PMID: 26745464 Review.
Cited by
-
The C-Terminus of Human Copper Importer Ctr1 Acts as a Binding Site and Transfers Copper to Atox1.Biophys J. 2016 Jan 5;110(1):95-102. doi: 10.1016/j.bpj.2015.11.016. Biophys J. 2016. PMID: 26745413 Free PMC article.
-
Strategies to combat cancer drug resistance: focus on copper metabolism and cuproptosis.Cancer Drug Resist. 2025 Mar 26;8:15. doi: 10.20517/cdr.2025.41. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40201308 Free PMC article. Review.
-
Cisplatin inhibits MEK1/2.Oncotarget. 2015 Sep 15;6(27):23510-22. doi: 10.18632/oncotarget.4355. Oncotarget. 2015. PMID: 26155939 Free PMC article.
-
Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein.Nat Commun. 2019 Jan 14;10(1):186. doi: 10.1038/s41467-018-08102-z. Nat Commun. 2019. PMID: 30643139 Free PMC article.
-
Mechanical stress promotes cisplatin-induced hepatocellular carcinoma cell death.Biomed Res Int. 2015;2015:430569. doi: 10.1155/2015/430569. Epub 2015 Jan 22. Biomed Res Int. 2015. PMID: 25685789 Free PMC article.
References
-
- Köberle B, Tomicic MT, Usanova S, Kaina B (2010) Cisplatin resistance: Preclinical findings and clinical implications. Biochim Biophys Acta, Rev Cancer 1806: 172–182. - PubMed
-
- Wheate NJ, Walker S, Craig GE, Oun R (2010) The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 39: 8113–8127. - PubMed
-
- Holzer AK, Howell SB (2006) The internalization and degradation of human copper transporter 1 following Cisplatin exposure. Cancer Res 66: 10944–10952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

