Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart
- PMID: 23936211
- PMCID: PMC3723823
- DOI: 10.1371/journal.pone.0070479
Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart
Abstract
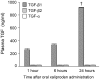
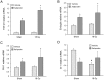
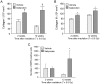
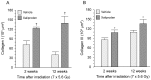
Radiation therapy in the treatment of cancer is dose limited by radiation injury in normal tissues such as the intestine and the heart. To identify the mechanistic involvement of transforming growth factor-beta 1 (TGF-β1) in intestinal and cardiac radiation injury, we studied the influence of pharmacological induction of TGF-β1 with xaliproden (SR 57746A) in rat models of radiation enteropathy and radiation-induced heart disease (RIHD). Because it was uncertain to what extent TGF-β induction may enhance radiation injury in heart and intestine, animals were exposed to irradiation schedules that cause mild to moderate (acute) radiation injury. In the radiation enteropathy model, male Sprague-Dawley rats received local irradiation of a 4-cm loop of rat ileum with 7 once-daily fractions of 5.6 Gy, and intestinal injury was assessed at 2 weeks and 12 weeks after irradiation. In the RIHD model, male Sprague-Dawley rats received local heart irradiation with a single dose of 18 Gy and were followed for 6 months after irradiation. Rats were treated orally with xaliproden starting 3 days before irradiation until the end of the experiments. Treatment with xaliproden increased circulating TGF-β1 levels by 300% and significantly induced expression of TGF-β1 and TGF-β1 target genes in the irradiated intestine and heart. Various radiation-induced structural changes in the intestine at 2 and 12 weeks were significantly enhanced with TGF-β1 induction. Similarly, in the RIHD model induction of TGF-β1 augmented radiation-induced changes in cardiac function and myocardial fibrosis. These results lend further support for the direct involvement of TGF-β1 in biological mechanisms of radiation-induced adverse remodeling in the intestine and the heart.
Conflict of interest statement
Figures



References
-
- Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW (2007) Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care 1: 23–29. - PubMed
-
- Theis VS, Sripadam R, Ramani V, Lal S (2010) Chronic radiation enteritis. Clin Oncol 22: 70–83. - PubMed
-
- Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE (2003) Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 45: 55–75. - PubMed
-
- Early Breast Cancer Trialists Collaborative Group (2000) Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 355: 1757–1770. - PubMed
-
- Darby SC, McGale P, Taylor CW, Peto R (2005) Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 6: 557–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

