Role of reactive oxygen species in the radiation response of human hematopoietic stem/progenitor cells
- PMID: 23936220
- PMCID: PMC3723682
- DOI: 10.1371/journal.pone.0070503
Role of reactive oxygen species in the radiation response of human hematopoietic stem/progenitor cells
Abstract
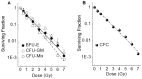
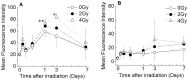
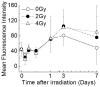
Hematopoietic stem/progenitor cells (HSPCs), which are present in small numbers in hematopoietic tissues, can differentiate into all hematopoietic lineages and self-renew to maintain their undifferentiated phenotype. HSPCs are extremely sensitive to oxidative stressors such as anti-cancer agents, radiation, and the extensive accumulation of reactive oxygen species (ROS). The quiescence and stemness of HSPCs are maintained by the regulation of mitochondrial biogenesis, ROS, and energy homeostasis in a special microenvironment called the stem cell niche. The present study evaluated the relationship between the production of intracellular ROS and mitochondrial function during the proliferation and differentiation of X-irradiated CD34(+) cells prepared from human placental/umbilical cord blood HSPCs. Highly purified CD34(+) HSPCs exposed to X-rays were cultured in liquid and semi-solid medium supplemented with hematopoietic cytokines. X-irradiated CD34(+) HSPCs treated with hematopoietic cytokines, which promote their proliferation and differentiation, exhibited dramatically suppressed cell growth and clonogenic potential. The amount of intracellular ROS in X-irradiated CD34(+) HSPCs was significantly higher than that in non-irradiated cells during the culture period. However, neither the intracellular mitochondrial content nor the mitochondrial superoxide production was elevated in X-irradiated CD34(+) HSPCs compared with non-irradiated cells. Radiation-induced gamma-H2AX expression was observed immediately following exposure to 4 Gy of X-rays and gradually decreased during the culture period. This study reveals that X-irradiation can increase persistent intracellular ROS in human CD34(+) HSPCs, which may not result from mitochondrial ROS due to mitochondrial dysfunction, and indicates that substantial DNA double-strand breakage can critically reduce the stem cell function.
Conflict of interest statement
Figures


References
-
- Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, et al. (2007) Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 7: 106–118. - PubMed
-
- Pervaiz S, Taneja R, Ghaffari S (2009) Oxidative Stress Regulation of Stem and Progenitor Cells. Antioxid Redox Signal 11: 2777–2789. - PubMed
-
- Zhou X, Li N, Wang Y, Wang Y, Zhang X, et al. (2011) Effects of X-irradiation on Mitochondrial DNA damage and its supercoiling formation change. Mitochondrion 11: 886–892. - PubMed
-
- Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

