Depth sensitivity and source-detector separations for near infrared spectroscopy based on the Colin27 brain template
- PMID: 23936292
- PMCID: PMC3731322
- DOI: 10.1371/journal.pone.0066319
Depth sensitivity and source-detector separations for near infrared spectroscopy based on the Colin27 brain template
Abstract
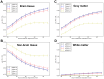
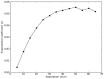
Understanding the spatial and depth sensitivity of non-invasive near-infrared spectroscopy (NIRS) measurements to brain tissue-i.e., near-infrared neuromonitoring (NIN) - is essential for designing experiments as well as interpreting research findings. However, a thorough characterization of such sensitivity in realistic head models has remained unavailable. In this study, we conducted 3,555 Monte Carlo (MC) simulations to densely cover the scalp of a well-characterized, adult male template brain (Colin27). We sought to evaluate: (i) the spatial sensitivity profile of NIRS to brain tissue as a function of source-detector separation, (ii) the NIRS sensitivity to brain tissue as a function of depth in this realistic and complex head model, and (iii) the effect of NIRS instrument sensitivity on detecting brain activation. We found that increasing the source-detector (SD) separation from 20 to 65 mm provides monotonic increases in sensitivity to brain tissue. For every 10 mm increase in SD separation (up to ~45 mm), sensitivity to gray matter increased an additional 4%. Our analyses also demonstrate that sensitivity in depth (S) decreases exponentially, with a "rule-of-thumb" formula S=0.75*0.85(depth). Thus, while the depth sensitivity of NIRS is not strictly limited, NIN signals in adult humans are strongly biased towards the outermost 10-15 mm of intracranial space. These general results, along with the detailed quantitation of sensitivity estimates around the head, can provide detailed guidance for interpreting the likely sources of NIRS signals, as well as help NIRS investigators design and plan better NIRS experiments, head probes and instruments.
Conflict of interest statement
Figures





References
-
- Villringer A, Chance B (1997) Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442. - PubMed
-
- Strangman G, Boas DA, Sutton JP (2002) Non-invasive neuroimaging using near-infrared light. Biol Psychiatry 52: 679–693. - PubMed
-
- Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29: 463–487. - PubMed
-
- Pogue BW, Patterson MS (1994) Frequency-domain optical absorption spectroscopy of finite tissue volumes using diffusion theory. Physics in medicine and biology 39: 25. - PubMed
-
- Moulton JD (1990) Diffusion modelling of picosecond laser pulse propagation in turbid media: McMaster University.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

