Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease
- PMID: 23936403
- PMCID: PMC3731353
- DOI: 10.1371/journal.pone.0070274
Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease
Abstract
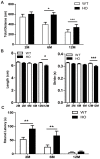
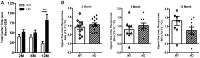
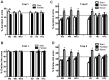
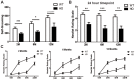
Parkinson's disease (PD) pathology is characterized by the formation of intra-neuronal inclusions called Lewy bodies, which are comprised of alpha-synuclein (α-syn). Duplication, triplication or genetic mutations in α-syn (A53T, A30P and E46K) are linked to autosomal dominant PD; thus implicating its role in the pathogenesis of PD. In both PD patients and mouse models, there is increasing evidence that neuronal dysfunction occurs before the accumulation of protein aggregates (i.e., α-syn) and neurodegeneration. Characterization of the timing and nature of symptomatic dysfunction is important for understanding the impact of α-syn on disease progression. Furthermore, this knowledge is essential for identifying pathways and molecular targets for therapeutic intervention. To this end, we examined various functional and morphological endpoints in the transgenic mouse model expressing the human A53T α-syn variant directed by the mouse prion promoter at specific ages relating to disease progression (2, 6 and 12 months of age). Our findings indicate A53T mice develop fine, sensorimotor, and synaptic deficits before the onset of age-related gross motor and cognitive dysfunction. Results from open field and rotarod tests show A53T mice develop age-dependent changes in locomotor activity and reduced anxiety-like behavior. Additionally, digigait analysis shows these mice develop an abnormal gait by 12 months of age. A53T mice also exhibit spatial memory deficits at 6 and 12 months, as demonstrated by Y-maze performance. In contrast to gross motor and cognitive changes, A53T mice display significant impairments in fine- and sensorimotor tasks such as grooming, nest building and acoustic startle as early as 1-2 months of age. These mice also show significant abnormalities in basal synaptic transmission, paired-pulse facilitation and long-term depression (LTD). Combined, these data indicate the A53T model exhibits early- and late-onset behavioral and synaptic impairments similar to PD patients and may provide useful endpoints for assessing novel therapeutic interventions for PD.
Conflict of interest statement
Figures


References
-
- George JM, Jin H, Woods WS, Clayton DF (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15: 361–372. - PubMed
-
- Clayton DF, George JM (1999) Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58: 120–129. - PubMed
-
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, et al. (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364: 1167–1169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

