Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3
- PMID: 23936628
- PMCID: PMC3738095
- DOI: 10.7554/eLife.00828
Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3
Abstract
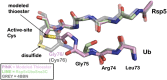
Ubiquitination by HECT E3 enzymes regulates myriad processes, including tumor suppression, transcription, protein trafficking, and degradation. HECT E3s use a two-step mechanism to ligate ubiquitin to target proteins. The first step is guided by interactions between the catalytic HECT domain and the E2∼ubiquitin intermediate, which promote formation of a transient, thioester-bonded HECT∼ubiquitin intermediate. Here we report that the second step of ligation is mediated by a distinct catalytic architecture established by both the HECT E3 and its covalently linked ubiquitin. The structure of a chemically trapped proxy for an E3∼ubiquitin-substrate intermediate reveals three-way interactions between ubiquitin and the bilobal HECT domain orienting the E3∼ubiquitin thioester bond for ligation, and restricting the location of the substrate-binding domain to prioritize target lysines for ubiquitination. The data allow visualization of an E2-to-E3-to-substrate ubiquitin transfer cascade, and show how HECT-specific ubiquitin interactions driving multiple reactions are repurposed by a major E3 conformational change to promote ligation. DOI:http://dx.doi.org/10.7554/eLife.00828.001.
Keywords: E2 conjugating enzyme; E3 ligase; HECT; NEDD4; Rsp5; S. cerevisiae; ubiquitin.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
















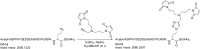
Comment in
-
Caught in the act.Elife. 2013 Aug 8;2:e01127. doi: 10.7554/eLife.01127. Elife. 2013. PMID: 23936629 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 5P30CA021765/CA/NCI NIH HHS/United States
- P41 GM103403/GM/NIGMS NIH HHS/United States
- R01 GM069530/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- 8P41 GM103403-10/GM/NIGMS NIH HHS/United States
- R01GM069530/GM/NIGMS NIH HHS/United States
- R01 GM073960/GM/NIGMS NIH HHS/United States
- P41 RR015301/RR/NCRR NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- 5P41RR015301-10/RR/NCRR NIH HHS/United States
- R01 GM058202/GM/NIGMS NIH HHS/United States
- R01GM073960/GM/NIGMS NIH HHS/United States
- 5R01 GM058202/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

