p38 MAPK Signaling in Pemphigus: Implications for Skin Autoimmunity
- PMID: 23936634
- PMCID: PMC3722958
- DOI: 10.1155/2013/728529
p38 MAPK Signaling in Pemphigus: Implications for Skin Autoimmunity
Abstract
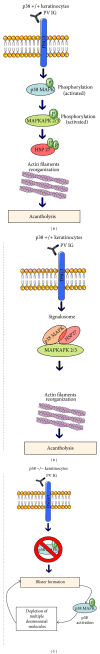
p38 mitogen activated protein kinase (p38 MAPK) signaling plays a major role in the modulation of immune-mediated inflammatory responses and therefore has been linked with several autoimmune diseases. The extent of the involvement of p38 MAPK in the pathogenesis of autoimmune blistering diseases has started to emerge, but whether it pays a critical role is a matter of debate. The activity of p38 MAPK has been studied in great detail during the loss of keratinocyte cell-cell adhesions and the development of pemphigus vulgaris (PV) and pemphigus foliaceus (PF). These diseases are characterised by autoantibodies targeting desmogleins (Dsg). Whether autoantibody-antigen interactions can trigger signaling pathways (such as p38 MAPK) that are tightly linked to the secretion of inflammatory mediators which may perpetuate inflammation and tissue damage in pemphigus remains unclear. Yet, the ability of p38 MAPK inhibitors to block activation of the proapoptotic proteinase caspase-3 suggests that the induction of apoptosis may be a consequence of p38 MAPK activation during acantholysis in PV. This review discusses the current evidence for the role of p38 MAPK in the pathogenesis of pemphigus. We will also present data relating to the targeting of these cascades as a means of therapeutic intervention.
Figures
References
-
- Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clinics in Dermatology. 2013;31(1):18–30. - PubMed
-
- Metz-Boutigue M, Shooshtarizadeh P, Prevost G, Haikel Y, Chich J. Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Current Pharmaceutical Design. 2010;16(9):1024–1039. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

