The power of integrating kinetic isotope effects into the formalism of the Michaelis-Menten equation
- PMID: 23937475
- PMCID: PMC4005594
- DOI: 10.1111/febs.12477
The power of integrating kinetic isotope effects into the formalism of the Michaelis-Menten equation
Abstract
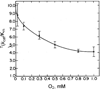
The final arbiter of enzyme mechanism is the ability to establish and test a kinetic mechanism. Isotope effects play a major role in expanding the scope and insight derived from the Michaelis-Menten equation. The integration of isotope effects into the formalism of the Michaelis-Menten equation began in the 1970s and has continued until the present. This review discusses a family of eukaryotic copper proteins, including dopamine β-monooxygenase, tyramine β-monooxygenase and peptidylglycine α-amidating enzyme, which are responsible for the synthesis of neuroactive compounds, norepinephrine, octopamine and C-terminally carboxamidated peptides, respectively. The review highlights the results of studies showing how combining kinetic isotope effects with initial rate parameters permits the evaluation of: (a) the order of substrate binding to multisubstrate enzymes; (b) the magnitude of individual rate constants in complex, multistep reactions; (c) the identification of chemical intermediates; and (d) the role of nonclassical (tunnelling) behaviour in C-H activation.
Keywords: enzymatic C-H activation; kinetic isotope effects; mechanism of enzyme action; mechanism of two copper monooxygenases; steady-state kinetics.
© 2013 FEBS.
Figures




References
-
- Michaelis L, Menten ML. The kinetics of the inversion effect. Biochem. Z. 1913;49:333–369.
-
- Monod J, Changeux JP, Jacob F. Allosteric proteins and cellular control systems. J. Mol. Biol. 1963;6:306-&. - PubMed
-
- Cleland WW. Kinetics of eyme-catalyzed reactions with 2 or more substrates or products .1. Nomenclature and rate equations. Biochim. Biophys. Acta. 1963;67:104-&. - PubMed
-
- Cleland WW. Kinetics ofenzyme-catalyzed reactions with 2 or more substrates or products .2. Inhibition, nomenclature and theory. Biochim. Biophys. Acta. 1963;67:173-&. - PubMed
-
- Cleland WW. Partition analysis and concept of net rate constants as tools in enzyme-kinetics. Biochemistry. 1975;14:3220–3224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

