Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function
- PMID: 23937663
- PMCID: PMC3898557
- DOI: 10.1111/cei.12186
Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function
Abstract
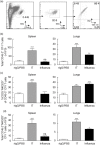
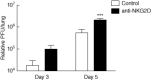
Primary viral infections induce activation of CD8(+) T cells responsible for effective resistance. We sought to characterize the nature of the CD8(+) T cell expansion observed after primary viral infection with influenza. Infection of naive mice with different strains of influenza resulted in the rapid expansion of memory CD8(+) T cells exhibiting a unique bystander phenotype with significant up-regulation of natural killer group 2D (NKG2D), but not CD25, on the CD44(high) CD8(+) T cells, suggesting an antigen non-specific phenotype. We further confirmed the non-specificity of this phenotype on ovalbumin-specific (OT-I) CD8(+) T cells, which are not specific to influenza. These non-specific CD8(+) T cells also displayed increased lytic capabilities and were observed primarily in the lung. Thus, influenza infection was shown to induce a rapid, antigen non-specific memory T cell expansion which is restricted to the specific site of inflammation. In contrast, CD8(+) T cells of a similar phenotype could be observed in other organs following administration of systemic agonistic anti-CD40 and interleukin-2 immunotherapy, demonstrating that bystander expansion in multiple sites is possible depending on whether the nature of activation is either acute or systemic. Finally, intranasal blockade of NKG2D resulted in a significant increase in viral replication early during the course of infection, suggesting that NKG2D is a critical mediator of anti-influenza responses prior to the initiation of adaptive immunity. These results characterize further the local bystander expansion of tissue-resident, memory CD8(+) T cells which, due to their early induction, may play an important NKG2D-mediated, antigen non-specific role during the early stages of viral infection.
Keywords: NKG2D; antigen non-specific; bystander activation; memory CD8; tissue-resident.
© 2013 British Society for Immunology.
Figures




References
-
- Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–5699. - PubMed
-
- Murata K, Garcia-Sastre A, Tsuji M, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. - PubMed
-
- Jacoby RO, Bhatt PN, Brownstein DG. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch Virol. 1989;108:49–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

