Mena/VASP and αII-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy
- PMID: 23937664
- PMCID: PMC3751641
- DOI: 10.1186/1478-811X-11-56
Mena/VASP and αII-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy
Abstract
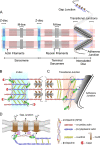
Background: In the heart, cytoplasmic actin networks are thought to have important roles in mechanical support, myofibrillogenesis, and ion channel function. However, subcellular localization of cytoplasmic actin isoforms and proteins involved in the modulation of the cytoplasmic actin networks are elusive. Mena and VASP are important regulators of actin dynamics. Due to the lethal phenotype of mice with combined deficiency in Mena and VASP, however, distinct cardiac roles of the proteins remain speculative. In the present study, we analyzed the physiological functions of Mena and VASP in the heart and also investigated the role of the proteins in the organization of cytoplasmic actin networks.
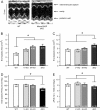
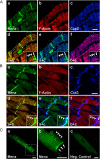
Results: We generated a mouse model, which simultaneously lacks Mena and VASP in the heart. Mena/VASP double-deficiency induced dilated cardiomyopathy and conduction abnormalities. In wild-type mice, Mena and VASP specifically interacted with a distinct αII-Spectrin splice variant (SH3i), which is in cardiomyocytes exclusively localized at Z- and intercalated discs. At Z- and intercalated discs, Mena and β-actin localized to the edges of the sarcomeres, where the thin filaments are anchored. In Mena/VASP double-deficient mice, β-actin networks were disrupted and the integrity of Z- and intercalated discs was markedly impaired.
Conclusions: Together, our data suggest that Mena, VASP, and αII-Spectrin assemble cardiac multi-protein complexes, which regulate cytoplasmic actin networks. Conversely, Mena/VASP deficiency results in disrupted β-actin assembly, Z- and intercalated disc malformation, and induces dilated cardiomyopathy and conduction abnormalities.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

