Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review
- PMID: 23937858
- PMCID: PMC3765173
- DOI: 10.1186/1471-2407-13-385
Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review
Abstract
Background: Current guidelines recommend anthracycline-based chemotherapy primarily with doxorubicin either as monotherapy or in combination with ifosfamide as the first-line treatment for most advanced STS subtypes. Therapeutic options after failure of doxorubicin and/or ifosfamide are limited. This study aimed to comprehensively review available data on the activity and safety of interventions in second- or later-line treatment of advanced STS.
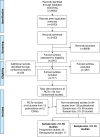
Methods: Electronic literature databases (Embase®, MEDLINE®, MEDLINE® In-Process, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews) were searched from 1980 to 01 March 2012 to identify randomised controlled trials (RCTs) and non-randomised studies (both prospective and retrospective) evaluating pharmacological interventions in patients with advanced STS pre-treated with anthracycline- and/or ifosfamide-based therapy.
Results: The review identified six RCTs (one phase III and five phase II trials) and 94 non-randomised studies. Based on the primary trial endpoints, RCTs demonstrated favourable efficacy for pazopanib over placebo (PFS: 4.6 months vs. 1.6 months), gemcitabine plus dacarbazine over dacarbazine monotherapy (3-month PFS rate: 54.2% vs. 35.2%), and trabectedin 3-weekly schedule over weekly schedule (TTP: 3.7 months vs. 2.3 months. The non-randomised studies demonstrated heterogeneity in efficacy and safety results.
Conclusions: Across the RCTs, pazopanib over placebo, gemcitabine-dacarbazine over dacarbazine, and trabectedin 3-weekly over weekly regimen clearly demonstrated a PFS advantage in the second- and later-line treatment of advanced STS. With only one phase III trial in this setting, there is a clear need for additional comparative trials to better understand the risk: benefit ratios of available agents and combinations.
Figures
References
-
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. - PubMed
-
- Jain A, Sajeevan KV, Babu KG, Lakshmaiah KC. Chemotherapy in adult soft tissue sarcoma. Indian J Cancer. 2009;46:274–287. - PubMed
-
- Weiss SW, Goldblum JR. In: Enzinger and Weiss's Soft Tissue Tumors. Weiss SW, Goldblum JR, editor. St Louis, Missouri: CV Mosby; 2001. General considerations; pp. 1–19.
-
- Spira AI, Ettinger DS. The use of chemotherapy in soft-tissue sarcomas. Oncologist. 2002;7:348–359. - PubMed
-
- Italiano A, Mathoulin-Pelissier S, Cesne AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre JM, Bui B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

