Dexamethasone reduces emesis after major gastrointestinal surgery (DREAMS)
- PMID: 23938028
- PMCID: PMC3765230
- DOI: 10.1186/1745-6215-14-249
Dexamethasone reduces emesis after major gastrointestinal surgery (DREAMS)
Abstract
Background: Postoperative nausea and vomiting is one of the most common complications affecting patients after surgery and causes significant morbidity and increased length of hospital stay. It is accepted that patients undergoing surgery on the bowel are at a higher risk. In the current era of minimally invasive colorectal surgery combined with enhanced recovery, reducing the incidence and severity of postoperative nausea and vomiting is particularly important. Dexamethasone is widely, but not universally used. It is known to improve appetite and gastric emptying, thus reduce vomiting. However, this benefit is not established in patients undergoing bowel surgery, and dexamethasone has possible side effects such as increased risk of wound infection and anastomotic leak that could adversely affect recovery.
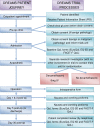
Design: DREAMS is a phase III, double-blind, multicenter, randomized controlled trial with the primary objective of determining if preoperative dexamethasone reduces postoperative nausea and vomiting in patients undergoing elective gastrointestinal resections. DREAMS aims to randomize 1,350 patients over 2.5 years.Patients undergoing laparoscopic or open colorectal resections for malignant or benign pathology are randomized between 8 mg intravenous dexamethasone and control (no dexamethasone). All patients are given one additional antiemetic at the time of induction, prior to randomization. Both the patient and their surgeon are blinded as to the treatment arm.Secondary objectives of the DREAMS trial are to determine whether there are other measurable benefits during recovery from surgery with the use of dexamethasone, including quicker return to oral diet and reduced length of stay. Health-related quality of life, fatigue and risks of infections will be investigated.
Trial registration: ISRCTN21973627.
Figures
References
-
- Macario A, Weigner M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89(3):652–658. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources