Pilot study assessing the feasibility of applying bilateral subthalamic nucleus deep brain stimulation in very early stage Parkinson's disease: study design and rationale
- PMID: 23938229
- PMCID: PMC4165487
- DOI: 10.3233/JPD-2012-012095
Pilot study assessing the feasibility of applying bilateral subthalamic nucleus deep brain stimulation in very early stage Parkinson's disease: study design and rationale
Abstract
Background: Deep brain stimulation provides significant symptomatic benefit for people with advanced Parkinson's disease whose symptoms are no longer adequately controlled with medication. Preliminary evidence suggests that subthalamic nucleus stimulation may also be efficacious in early Parkinson's disease, and results of animal studies suggest that it may spare dopaminergic neurons in the substantia nigra.
Objective: We report the methodology and design of a novel Phase I clinical trial testing the safety and tolerability of deep brain stimulation in early Parkinson's disease and discuss previous failed attempts at neuroprotection.
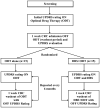
Methods: We recently conducted a prospective, randomized, parallel-group, single-blind pilot clinical trial of deep brain stimulation in early Parkinson's disease. Subjects were randomized to receive either optimal drug therapy or deep brain stimulation plus optimal drug therapy. Follow-up visits occurred every six months for a period of two years and included week-long therapy washouts.
Results: Thirty subjects with Hoehn & Yahr Stage II idiopathic Parkinson's disease were enrolled over a period of 32 months. Twenty-nine subjects completed all follow-up visits; one patient in the optimal drug therapy group withdrew from the study after baseline. Baseline characteristics for all thirty patients were not significantly different.
Conclusions: This study demonstrates that it is possible to recruit and retain subjects in a clinical trial testing deep brain stimulation in early Parkinson's disease. The results of this trial will be used to support the design of a Phase III, multicenter trial investigating the efficacy of deep brain stimulation in early Parkinson's disease.
Conflict of interest statement
Figures

Similar articles
-
Subthalamic Nucleus Deep Brain Stimulation May Reduce Medication Costs in Early Stage Parkinson's Disease.J Parkinsons Dis. 2016;6(1):125-31. doi: 10.3233/JPD-150712. J Parkinsons Dis. 2016. PMID: 26967937 Free PMC article. Clinical Trial.
-
Subthalamic nucleus deep brain stimulation in early stage Parkinson's disease.Parkinsonism Relat Disord. 2014 Jul;20(7):731-7. doi: 10.1016/j.parkreldis.2014.03.019. Epub 2014 Mar 28. Parkinsonism Relat Disord. 2014. PMID: 24768120 Free PMC article. Clinical Trial.
-
Parkinson's disease progression at 30 years: a study of subthalamic deep brain-stimulated patients.Brain. 2011 Jul;134(Pt 7):2074-84. doi: 10.1093/brain/awr121. Epub 2011 Jun 11. Brain. 2011. PMID: 21666262 Clinical Trial.
-
Suicide and suicide attempts after subthalamic nucleus stimulation in Parkinson's disease: a systematic review and meta-analysis.Neurol Sci. 2021 Jan;42(1):267-274. doi: 10.1007/s10072-020-04555-7. Epub 2020 Jul 8. Neurol Sci. 2021. PMID: 32643134
-
Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients With Parkinson's Disease: Executive Summary.Neurosurgery. 2018 Jun 1;82(6):753-756. doi: 10.1093/neuros/nyy037. Neurosurgery. 2018. PMID: 29538685 Free PMC article.
Cited by
-
Subthalamic Nucleus Deep Brain Stimulation May Reduce Medication Costs in Early Stage Parkinson's Disease.J Parkinsons Dis. 2016;6(1):125-31. doi: 10.3233/JPD-150712. J Parkinsons Dis. 2016. PMID: 26967937 Free PMC article. Clinical Trial.
-
Targeting for Success: Demonstrating Proof-of-Concept with Mechanistic Early Phase Clinical Pharmacology Studies for Disease-Modification in Neurodegenerative Disorders.Int J Mol Sci. 2021 Feb 5;22(4):1615. doi: 10.3390/ijms22041615. Int J Mol Sci. 2021. PMID: 33562713 Free PMC article. Review.
-
Subthalamic Nucleus Deep Brain Stimulation in Early Stage Parkinson's Disease Is Not Associated with Increased Body Mass Index.Parkinsons Dis. 2017;2017:7163801. doi: 10.1155/2017/7163801. Epub 2017 Jun 6. Parkinsons Dis. 2017. PMID: 28676842 Free PMC article.
-
Deep brain stimulation in early-stage Parkinson disease: Five-year outcomes.Neurology. 2020 Jul 28;95(4):e393-e401. doi: 10.1212/WNL.0000000000009946. Epub 2020 Jun 29. Neurology. 2020. PMID: 32601120 Free PMC article. Clinical Trial.
-
Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease.Neurology. 2018 Jul 31;91(5):e463-e471. doi: 10.1212/WNL.0000000000005903. Epub 2018 Jun 29. Neurology. 2018. PMID: 29959266 Free PMC article. Clinical Trial.
References
-
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. - PubMed
-
- Lim SY, Lang AE. The nonmotor symptoms of Parkinson's disease--an overview. Mov Disord. 25(Suppl 1):S123–30. - PubMed
-
- Salawu FK, Danburam A, Olokoba AB. Non-motor symptoms of Parkinson's disease: diagnosis and management. Niger J Med. 19:126–31. - PubMed
-
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc. 2004;52:784–8. - PubMed
-
- Olanow CW. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology. 2009;72:S59–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

