Pilot study assessing the feasibility of applying bilateral subthalamic nucleus deep brain stimulation in very early stage Parkinson's disease: study design and rationale
- PMID: 23938229
- PMCID: PMC4165487
- DOI: 10.3233/JPD-2012-012095
Pilot study assessing the feasibility of applying bilateral subthalamic nucleus deep brain stimulation in very early stage Parkinson's disease: study design and rationale
Abstract
Background: Deep brain stimulation provides significant symptomatic benefit for people with advanced Parkinson's disease whose symptoms are no longer adequately controlled with medication. Preliminary evidence suggests that subthalamic nucleus stimulation may also be efficacious in early Parkinson's disease, and results of animal studies suggest that it may spare dopaminergic neurons in the substantia nigra.
Objective: We report the methodology and design of a novel Phase I clinical trial testing the safety and tolerability of deep brain stimulation in early Parkinson's disease and discuss previous failed attempts at neuroprotection.
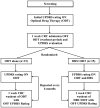
Methods: We recently conducted a prospective, randomized, parallel-group, single-blind pilot clinical trial of deep brain stimulation in early Parkinson's disease. Subjects were randomized to receive either optimal drug therapy or deep brain stimulation plus optimal drug therapy. Follow-up visits occurred every six months for a period of two years and included week-long therapy washouts.
Results: Thirty subjects with Hoehn & Yahr Stage II idiopathic Parkinson's disease were enrolled over a period of 32 months. Twenty-nine subjects completed all follow-up visits; one patient in the optimal drug therapy group withdrew from the study after baseline. Baseline characteristics for all thirty patients were not significantly different.
Conclusions: This study demonstrates that it is possible to recruit and retain subjects in a clinical trial testing deep brain stimulation in early Parkinson's disease. The results of this trial will be used to support the design of a Phase III, multicenter trial investigating the efficacy of deep brain stimulation in early Parkinson's disease.
Conflict of interest statement
Figures

References
-
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. - PubMed
-
- Lim SY, Lang AE. The nonmotor symptoms of Parkinson's disease--an overview. Mov Disord. 25(Suppl 1):S123–30. - PubMed
-
- Salawu FK, Danburam A, Olokoba AB. Non-motor symptoms of Parkinson's disease: diagnosis and management. Niger J Med. 19:126–31. - PubMed
-
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc. 2004;52:784–8. - PubMed
-
- Olanow CW. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology. 2009;72:S59–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

