Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis
- PMID: 23938324
- PMCID: PMC7119452
- DOI: 10.1016/j.ajpath.2013.06.023
Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis
Abstract
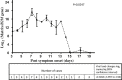
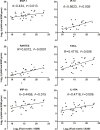
Pathological studies on fatal cases caused by 2009 pandemic influenza H1N1 virus (2009 pH1N1) reported extensive diffuse alveolar damage and virus infection predominantly in the lung parenchyma. However, the host immune response after severe 2009 pH1N1 infection is poorly understood. Herein, we investigated viral load, the immune response, and apoptosis in lung tissues from 50 fatal cases with 2009 pH1N1 virus infection. The results suggested that 7 of the 27 cytokines/chemokines showed remarkably high expression, including IL-1 receptor antagonist protein, IL-6, tumor necrosis factor-α, IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-β, and interferon-inducible protein-10 in lung tissues of 2009 pH1N1 fatal cases. Viral load, which showed the highest level on day 7 of illness onset and persisted until day 17 of illness, was positively correlated with mRNA levels of IL-1 receptor antagonist protein, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-β, interferon-inducible protein-10, and regulated on activation normal T-cell expressed and secreted. Apoptosis was evident in lung tissues stained by the TUNEL assay. Decreased Fas and elevated FasL mRNA levels were present in lung tissues, and cleaved caspase-3 was frequently seen in pneumocytes, submucosal glands, and lymphoid tissues. The pathogenesis of the 2009 pH1N1 virus infection is associated with viral replication and production of proinflammatory mediators. FasL and caspase-3 are involved in the pathway of 2009 pH1N1 virus-induced apoptosis in lung tissues, and the disequilibrium between the Fas and FasL level in lung tissues could contribute to delayed clearance of the virus and subsequent pathological damages.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Why does pandemic influenza virus kill?Am J Pathol. 2013 Oct;183(4):1125-1127. doi: 10.1016/j.ajpath.2013.06.020. Epub 2013 Aug 1. Am J Pathol. 2013. PMID: 23916382 Free PMC article.
References
-
- Zimmer S.M., Burke D.S. Historical perspective-emergence of influenza (H1N1) viruses. N Engl J Med. 2009;361:279–285. - PubMed
-
- Lurie N. H1N1 influenza, public health preparedness, and health care reform. N Engl J Med. 2009;361:843–845. - PubMed
-
- Mauad T., Hajjar L.A., Callegari G.D., da Silva L.F., Schout D., Galas F.R., Alves V.A., Malheiros D.M., Auler J.O., Jr., Ferreira A.F., Borsato M.R., Bezerra S.M., Gutierrez P.S., Caldini E.T., Pasqualucci C.A., Dolhnikoff M., Saldiva P.H. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181:72–79. - PubMed
-
- Okudaira M., Inui M., Nakamura N., Yoshimura S. Pathology of influenza, especially on the autopsy findings of seven cases of influenza A2 virus infection during the pandemic at the beginning of 1962. Acta Pathol Jpn. 1963;13:119–122. - PubMed
-
- Oseasohn R., Adelson L., Kaji M. Clinicopathologic study of thirty-three fatal cases of Asian influenza. N Engl J Med. 1959;260:509–518. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

