Implications of autophagy for vascular smooth muscle cell function and plasticity
- PMID: 23938401
- PMCID: PMC3859773
- DOI: 10.1016/j.freeradbiomed.2013.08.003
Implications of autophagy for vascular smooth muscle cell function and plasticity
Abstract
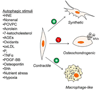
Vascular smooth muscle cells (VSMCs) are fundamental in regulating blood pressure and distributing oxygen and nutrients to peripheral tissues. They also possess remarkable plasticity, with the capacity to switch to synthetic, macrophage-like, or osteochondrogenic phenotypes when cued by external stimuli. In arterial diseases such as atherosclerosis and restenosis, this plasticity seems to be critical and, depending on the disease context, can be deleterious or beneficial. Therefore, understanding the mechanisms regulating VSMC phenotype and survival is essential for developing new therapies for vascular disease as well as understanding how secondary complications due to surgical interventions develop. In this regard, the cellular process of autophagy is increasingly being recognized as a major player in vascular biology and a critical determinant of VSMC phenotype and survival. Although autophagy was identified in lesional VSMCs in the 1960s, our understanding of the implications of autophagy in arterial diseases and the stimuli promoting its activation in VSMCs is only now being elucidated. In this review, we highlight the evidence for autophagy occurring in VSMCs in vivo, elaborate on the stimuli and processes regulating autophagy, and discuss the current understanding of the role of autophagy in vascular disease.
Keywords: Atherosclerosis; Cardiovascular; Free radicals; Hypertension; Oxidative stress; Proliferation; Restenosis.
Copyright © 2013. Published by Elsevier Inc.
Figures

References
-
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. - PubMed
-
- Orford JL, Selwyn AP, Ganz P, Popma P, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol. 2000;86:6H–11H. - PubMed
-
- Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–1867. - PubMed
-
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

