Linking spermatid ribonucleic acid (RNA) binding protein and retrogene diversity to reproductive success
- PMID: 23938467
- PMCID: PMC3820935
- DOI: 10.1074/mcp.M113.030585
Linking spermatid ribonucleic acid (RNA) binding protein and retrogene diversity to reproductive success
Abstract
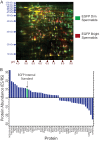
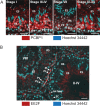
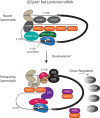
Spermiogenesis is a postmeiotic process that drives development of round spermatids into fully elongated spermatozoa. Spermatid elongation is largely controlled post-transcriptionally after global silencing of mRNA synthesis from the haploid genome. Here, rats that differentially express EGFP from a lentiviral transgene during early and late steps of spermiogenesis were used to flow sort fractions of round and elongating spermatids. Mass-spectral analysis of 2D gel protein spots enriched >3-fold in each fraction revealed a heterogeneous RNA binding proteome (hnRNPA2/b1, hnRNPA3, hnRPDL, hnRNPK, hnRNPL, hnRNPM, PABPC1, PABPC4, PCBP1, PCBP3, PTBP2, PSIP1, RGSL1, RUVBL2, SARNP2, TDRD6, TDRD7) abundantly expressed in round spermatids prior to their elongation. Notably, each protein within this ontology cluster regulates alternative splicing, sub-cellular transport, degradation and/or translational repression of mRNAs. In contrast, elongating spermatid fractions were enriched with glycolytic enzymes, redox enzymes and protein synthesis factors. Retrogene-encoded proteins were over-represented among the most abundant elongating spermatid factors identified. Consistent with these biochemical activities, plus corresponding histological profiles, the identified RNA processing factors are predicted to collectively drive post-transcriptional expression of an alternative exome that fuels finishing steps of sperm maturation and fitness.
Figures



References
-
- Zhu H., Shyh-Chang N., Segre A. V., Shinoda G., Shah S. P., Einhorn W. S., Takeuchi A., Engreitz J. M., Hagan J. P., Kharas M. G., Urbach A., Thornton J. E., Triboulet R., Gregory R. I., Altshuler D., Daley G. Q. (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

