When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses
- PMID: 23938758
- PMCID: PMC3758193
- DOI: 10.1098/rstb.2013.0051
When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses
Abstract
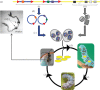
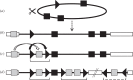
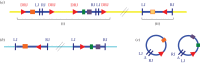
The Polydnaviridae (PDV), including the Bracovirus (BV) and Ichnovirus genera, originated from the integration of unrelated viruses in the genomes of two parasitoid wasp lineages, in a remarkable example of convergent evolution. Functionally active PDVs represent the most compelling evolutionary success among endogenous viral elements (EVEs). BV evolved from the domestication by braconid wasps of a nudivirus 100 Ma. The nudivirus genome has become an EVE involved in BV particle production but is not encapsidated. Instead, BV genomes have co-opted virulence genes, used by the wasps to control the immunity and development of their hosts. Gene transfers and duplications have shaped BV genomes, now encoding hundreds of genes. Phylogenomic studies suggest that BVs contribute largely to wasp diversification and adaptation to their hosts. A genome evolution model explains how multidirectional wasp adaptation to different host species could have fostered PDV genome extension. Integrative studies linking ecological data on the wasp to genomic analyses should provide new insights into the adaptive role of particular BV genes. Forthcoming genomic advances should also indicate if the associations between endoparasitoid wasps and symbiotic viruses evolved because of their particularly intimate interactions with their hosts, or if similar domesticated EVEs could be uncovered in other parasites.
Keywords: Cotesia; genome evolution; obligatory mutualism; parasitoid wasp; polydnavirus; virus adaptation.
Figures


References
-
- Emerman M, Malik HS. 2010. Paleovirology: modern consequences of ancient viruses. PLoS Biol. 8, e1000301 (doi:10.1371/journal.pbio.1000301) - DOI - PMC - PubMed
-
- Thézé J, Bézier A, Periquet G, Drezen J-M, Herniou EA. 2011. Paleozoic origin of insect large dsDNA viruses. Proc. Natl Acad. Sci. USA 108, 15 931–15 935 (doi:10.1073/pnas.1105580108) - DOI - PMC - PubMed
-
- Katzourakis A, Gifford RJ, Tristem M, Gilbert MT, Pybus OG. 2009. Macroevolution of complex retroviruses. Science 325, 1512 (doi:10.1126/science.1174149) - DOI - PubMed
-
- Katzourakis A, Gifford RJ. 2010. Endogenous viral elements in animal genomes. PLoS Genet. 6, e1001191 (doi:10.1371/journal.pgen.1001191) - DOI - PMC - PubMed
-
- Strand MR, Burke GR. 2012. Polydnaviruses as symbionts and gene delivery systems. PLoS Pathog. 8, e1002757 (doi:10.1371/journal.ppat.1002757) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
