Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective
- PMID: 23939427
- PMCID: PMC3759924
- DOI: 10.3390/ijms140816532
Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective
Abstract
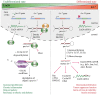
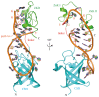
Lin28 is an essential RNA-binding protein that is ubiquitously expressed in embryonic stem cells. Its physiological function has been linked to the regulation of differentiation, development, and oncogenesis as well as glucose metabolism. Lin28 mediates these pleiotropic functions by inhibiting let-7 miRNA biogenesis and by modulating the translation of target mRNAs. Both activities strongly depend on Lin28's RNA-binding domains (RBDs), an N-terminal cold-shock domain (CSD) and a C-terminal Zn-knuckle domain (ZKD). Recent biochemical and structural studies revealed the mechanisms of how Lin28 controls let-7 biogenesis. Lin28 binds to the terminal loop of pri- and pre-let-7 miRNA and represses their processing by Drosha and Dicer. Several biochemical and structural studies showed that the specificity of this interaction is mainly mediated by the ZKD with a conserved GGAGA or GGAGA-like motif. Further RNA crosslinking and immunoprecipitation coupled to high-throughput sequencing (CLIP-seq) studies confirmed this binding motif and uncovered a large number of new mRNA binding sites. Here we review exciting recent progress in our understanding of how Lin28 binds structurally diverse RNAs and fulfills its pleiotropic functions.
Figures






References
-
- Ambros V., Horvitz H. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. - PubMed
-
- Moss E.G., Lee R.C., Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. - PubMed
-
- Seggerson K., Tang L., Moss E.G. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev. Biol. 2002;243:215–225. - PubMed
-
- Moss E.G., Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev. Biol. 2003;258:432–442. - PubMed
-
- Darr H., Benvenisty N. Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem Cells. 2009;27:352–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

