The severe autosomal dominant retinitis pigmentosa rhodopsin mutant Ter349Glu mislocalizes and induces rapid rod cell death
- PMID: 23940033
- PMCID: PMC3790004
- DOI: 10.1074/jbc.M113.495184
The severe autosomal dominant retinitis pigmentosa rhodopsin mutant Ter349Glu mislocalizes and induces rapid rod cell death
Abstract
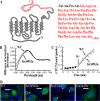
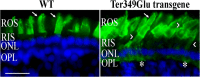
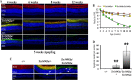
Mutations in the rhodopsin gene cause approximately one-tenth of retinitis pigmentosa cases worldwide, and most result in endoplasmic reticulum retention and apoptosis. Other rhodopsin mutations cause receptor mislocalization, diminished/constitutive activity, or faulty protein-protein interactions. The purpose of this study was to test for mechanisms by which the autosomal dominant rhodopsin mutation Ter349Glu causes an early, rapid retinal degeneration in patients. The mutation adds an additional 51 amino acids to the C terminus of the protein. Folding and ligand interaction of Ter349Glu rhodopsin were tested by ultraviolet-visible (UV-visible) spectrophotometry. The ability of the mutant to initiate phototransduction was tested using a radioactive filter binding assay. Photoreceptor localization was assessed both in vitro and in vivo utilizing fluorescent immunochemistry on transfected cells, transgenic Xenopus laevis, and knock-in mice. Photoreceptor ultrastructure was observed by transmission electron microscopy. Spectrally, Ter349Glu rhodopsin behaves similarly to wild-type rhodopsin, absorbing maximally at 500 nm. The mutant protein also displays in vitro G protein activation similar to that of WT. In cultured cells, mislocalization was observed at high expression levels whereas ciliary localization occurred at low expression levels. Similarly, transgenic X. laevis expressing Ter349Glu rhodopsin exhibited partial mislocalization. Analysis of the Ter349Glu rhodopsin knock-in mouse showed a rapid, early onset degeneration in homozygotes with a loss of proper rod outer segment development and improper disc formation. Together, the data show that both mislocalization and rod outer segment morphogenesis are likely associated with the human phenotype.
Keywords: Cilia; G Protein-coupled Receptors (GPCRs); Neurodegeneration; Neurodegenerative Diseases; Photoreceptors; Phototransduction; Retinal Degeneration; Rhodopsin; Ter349Glu; Vision.
Figures


References
-
- al-Maghtheh M., Gregory C., Inglehearn C., Hardcastle A., Bhattacharya S. (1993) Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Hum. Mutat. 2, 249–255 - PubMed
-
- Hollingsworth T. J., Gross A. K. (2012) Defective trafficking of rhodopsin and its role in retinal degenerations. Int. Rev. Cell Mol. Biol. 293, 1–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

