The allosteric role of the AAA+ domain of ChlD protein from the magnesium chelatase of synechocystis species PCC 6803
- PMID: 23940041
- PMCID: PMC3789969
- DOI: 10.1074/jbc.M113.477943
The allosteric role of the AAA+ domain of ChlD protein from the magnesium chelatase of synechocystis species PCC 6803
Abstract
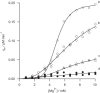
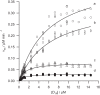
Magnesium chelatase is an AAA(+) ATPase that catalyzes the first step in chlorophyll biosynthesis, the energetically unfavorable insertion of a magnesium ion into a porphyrin ring. This enzyme contains two AAA(+) domains, one active in the ChlI protein and one inactive in the ChlD protein. Using a series of mutants in the AAA(+) domain of ChlD, we show that this site is essential for magnesium chelation and allosterically regulates Mg(2+) and MgATP(2-) binding.
Keywords: ATPases; Biosynthesis; Enzyme Catalysis; Mutagenesis Site-specific; Porphyrin.
Figures
References
-
- Reid J. D., Hunter C. N. (2004) Magnesium-dependent ATPase activity and cooperativity of magnesium chelatase from Synechocystis sp. PCC6803. J. Biol. Chem. 279, 26893–26899 - PubMed
-
- Gibson L. C., Willows R. D., Kannangara C. G., von Wettstein D., Hunter C. N. (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitiution of activity by combining the products of the bchH, -I and -D genes expressed in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 92, 1941–1944 - PMC - PubMed
-
- Jensen P. E., Gibson L. C., Henningsen K. W., Hunter C. N. (1996) Expression of the chlI, chlD, and chlH genes from the cyanobacterium Synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. J. Biol. Chem. 271, 16662–16667 - PubMed
-
- Willows R. D., Gibson L. C., Kanangara C. G., Hunter C. N., von Wettstein D. (1996) Three separate proteins constitute the magnesium chelatase of Rhodobacter sphaeroides. Eur. J. Biochem. 235, 438–443 - PubMed
-
- Karger G. A., Reid J. D., Hunter C. N. (2001) Characterization of the binding of deuteroporphyrin IX to the magnesium chelatase H subunit and spectroscopic properties of the complex. Biochemistry 40, 9291–9299 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

