hGAAP promotes cell adhesion and migration via the stimulation of store-operated Ca2+ entry and calpain 2
- PMID: 23940116
- PMCID: PMC3747308
- DOI: 10.1083/jcb.201301016
hGAAP promotes cell adhesion and migration via the stimulation of store-operated Ca2+ entry and calpain 2
Abstract
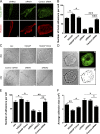
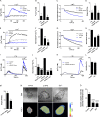
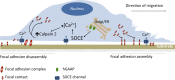
Golgi antiapoptotic proteins (GAAPs) are highly conserved Golgi membrane proteins that inhibit apoptosis and promote Ca(2+) release from intracellular stores. Given the role of Ca(2+) in controlling cell adhesion and motility, we hypothesized that human GAAP (hGAAP) might influence these events. In this paper, we present evidence that hGAAP increased cell adhesion, spreading, and migration in a manner that depended on the C-terminal domain of hGAAP. We show that hGAAP increased store-operated Ca(2+) entry and thereby the activity of calpain at newly forming protrusions. These hGAAP-dependent effects regulated focal adhesion dynamics and cell migration. Indeed, inhibition or knockdown of calpain 2 abrogated the effects of hGAAP on cell spreading and migration. Our data reveal that hGAAP is a novel regulator of focal adhesion dynamics, cell adhesion, and migration by controlling localized Ca(2+)-dependent activation of calpain.
Figures





Similar articles
-
Stimulation of cell invasion by the Golgi Ion Channel GAAP/TMBIM4 via an H2O2-Dependent Mechanism.Redox Biol. 2020 Jan;28:101361. doi: 10.1016/j.redox.2019.101361. Epub 2019 Oct 22. Redox Biol. 2020. PMID: 31693977 Free PMC article.
-
Human and viral Golgi anti-apoptotic proteins (GAAPs) oligomerize via different mechanisms and monomeric GAAP inhibits apoptosis and modulates calcium.J Biol Chem. 2013 May 3;288(18):13057-67. doi: 10.1074/jbc.M112.414367. Epub 2013 Mar 18. J Biol Chem. 2013. PMID: 23508950 Free PMC article.
-
The US21 viroporin of human cytomegalovirus stimulates cell migration and adhesion.mBio. 2023 Aug 31;14(4):e0074923. doi: 10.1128/mbio.00749-23. Epub 2023 Jul 21. mBio. 2023. PMID: 37477430 Free PMC article.
-
Golgi anti-apoptotic protein: a tale of camels, calcium, channels and cancer.Open Biol. 2017 May;7(5):170045. doi: 10.1098/rsob.170045. Open Biol. 2017. PMID: 28469007 Free PMC article. Review.
-
Evidence for calpains in cancer metastasis.J Cell Physiol. 2019 Jun;234(6):8233-8240. doi: 10.1002/jcp.27649. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370545 Review.
Cited by
-
Bcl-2 proteins and calcium signaling: complexity beneath the surface.Oncogene. 2016 Sep 29;35(39):5079-92. doi: 10.1038/onc.2016.31. Epub 2016 Mar 14. Oncogene. 2016. PMID: 26973249 Review.
-
Endothelial cell calpain as a critical modulator of angiogenesis.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1326-1335. doi: 10.1016/j.bbadis.2017.03.021. Epub 2017 Mar 30. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28366876 Free PMC article. Review.
-
Ectromelia Virus Affects the Formation and Spatial Organization of Adhesive Structures in Murine Dendritic Cells In Vitro.Int J Mol Sci. 2023 Dec 31;25(1):558. doi: 10.3390/ijms25010558. Int J Mol Sci. 2023. PMID: 38203729 Free PMC article.
-
The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells.Redox Biol. 2019 Jan;20:367-378. doi: 10.1016/j.redox.2018.10.016. Epub 2018 Oct 25. Redox Biol. 2019. PMID: 30408752 Free PMC article.
-
Amentoflavone regulates the miR-124-3p/CAPN2 axis to promote mitochondrial autophagy in HCC cells.Toxicol Res (Camb). 2024 Jul 23;13(4):tfae110. doi: 10.1093/toxres/tfae110. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39050595 Free PMC article.
References
-
- Carrara G., Saraiva N., Gubser C., Johnson B.F., Smith G.L. 2012. Six-transmembrane topology for Golgi anti-apoptotic protein (GAAP) and Bax inhibitor 1 (BI-1) provides model for the transmembrane Bax inhibitor-containing motif (TMBIM) family. J. Biol. Chem. 287:15896–15905 10.1074/jbc.M111.336149 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

