Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma
- PMID: 23940216
- PMCID: PMC4021043
- DOI: 10.1200/JCO.2012.47.2464
Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma
Abstract
Purpose: A randomized, phase III, placebo-controlled, partially blinded clinical trial (REGAL [Recent in in Glioblastoma Alone and With Lomustine]) was conducted to determine the efficacy of cediranib, an oral pan-vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, either as monotherapy or in combination with lomustine versus lomustine in patients with recurrent glioblastoma.
Patients and methods: Patients (N = 325) with recurrent glioblastoma who previously received radiation and temozolomide were randomly assigned 2:2:1 to receive (1) cediranib (30 mg) monotherapy; (2) cediranib (20 mg) plus lomustine (110 mg/m(2)); (3) lomustine (110 mg/m(2)) plus a placebo. The primary end point was progression-free survival based on blinded, independent radiographic assessment of postcontrast T1-weighted and noncontrast T2-weighted magnetic resonance imaging (MRI) brain scans.
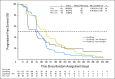
Results: The primary end point of progression-free survival (PFS) was not significantly different for either cediranib alone (hazard ratio [HR] = 1.05; 95% CI, 0.74 to 1.50; two-sided P = .90) or cediranib in combination with lomustine (HR = 0.76; 95% CI, 0.53 to 1.08; two-sided P = .16) versus lomustine based on independent or local review of postcontrast T1-weighted MRI.
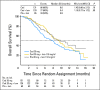
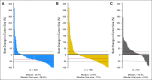
Conclusion: This study did not meet its primary end point of PFS prolongation with cediranib either as monotherapy or in combination with lomustine versus lomustine in patients with recurrent glioblastoma, although cediranib showed evidence of clinical activity on some secondary end points including time to deterioration in neurologic status and corticosteroid-sparing effects.
Trial registration: ClinicalTrials.gov NCT00777153.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Reply to M.C. Chamberlain.J Clin Oncol. 2014 Jul 20;32(21):2273-4. doi: 10.1200/JCO.2013.54.2878. Epub 2014 Jun 16. J Clin Oncol. 2014. PMID: 24934784 No abstract available.
-
Is there a role for vascular endothelial growth factor receptor 2 inhibitors in glioblastoma?J Clin Oncol. 2014 Jul 20;32(21):2272. doi: 10.1200/JCO.2013.54.0088. Epub 2014 Jun 16. J Clin Oncol. 2014. PMID: 24934790 No abstract available.
References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. - PubMed
-
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. - PubMed
-
- Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

