Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer
- PMID: 23940286
- PMCID: PMC3776260
- DOI: 10.1093/jnci/djt205
Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer
Abstract
Background: There is a medical need for diagnostic biomarkers in lung cancer. We evaluated the diagnostic performance of complement activation fragments.
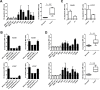
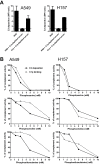
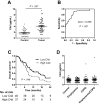
Methods: We assessed complement activation in four bronchial epithelial and seven lung cancer cell lines. C4d, a degradation product of complement activation, was determined in 90 primary lung tumors; bronchoalveolar lavage supernatants from patients with lung cancer (n = 50) and nonmalignant respiratory diseases (n = 22); and plasma samples from advanced (n = 50) and early lung cancer patients (n = 84) subjects with inflammatory lung diseases (n = 133), and asymptomatic individuals enrolled in a lung cancer computed tomography screening program (n = 190). Two-sided P values were calculated by Mann-Whitney U test.
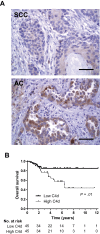
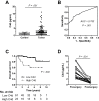
Results: Lung cancer cells activated the classical complement pathway mediated by C1q binding that was inhibited by phosphomonoesters. Survival was decreased in patients with high C4d deposition in tumors (hazard ratio [HR] = 3.06; 95% confidence interval [CI] = 1.18 to 7.91). C4d levels were increased in bronchoalveolar lavage fluid from lung cancer patients compared with patients with nonmalignant respiratory diseases (0.61 ± 0.87 vs 0.16 ± 0.11 µg/mL; P < .001). C4d levels in plasma samples from lung cancer patients at both advanced and early stages were also increased compared with control subjects (4.13 ± 2.02 vs 1.86 ± 0.95 µg/mL, P < 0.001; 3.18 ± 3.20 vs 1.13 ± 0.69 µg/mL, P < .001, respectively). C4d plasma levels were associated with shorter survival in patients at advanced (HR = 1.59; 95% CI = 0.97 to 2.60) and early stages (HR = 5.57; 95% CI = 1.60 to 19.39). Plasma C4d levels were reduced after surgical removal of lung tumors (P < .001) and were associated with increased lung cancer risk in asymptomatic individuals with (n = 32) or without lung cancer (n = 158) (odds ratio = 4.38; 95% CI = 1.61 to 11.93).
Conclusions: Complement fragment C4d may serve as a biomarker for early diagnosis and prognosis of lung cancer.
Figures


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics., 2013CA Cancer J Clin. 2013;63(1):11–30 - PubMed
-
- Finn OJ. Immune response as a biomarker for cancer detection and a lot more. N Engl J Med. 2005;353(12):1288–1290 - PubMed
-
- Shepherd FA, Douillard JY, Blumenschein GR., Jr Immunotherapy for non-small cell lung cancer: novel approaches to improve patient outcome. J Thorac Oncol. 2011;6(10):1763–1773 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

