Surge of neurophysiological coherence and connectivity in the dying brain
- PMID: 23940340
- PMCID: PMC3761619
- DOI: 10.1073/pnas.1308285110
Surge of neurophysiological coherence and connectivity in the dying brain
Abstract
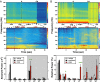
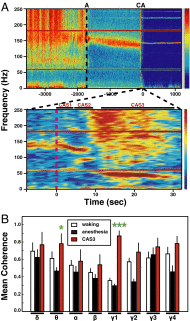
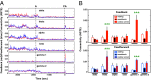
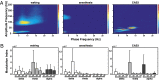
The brain is assumed to be hypoactive during cardiac arrest. However, the neurophysiological state of the brain immediately following cardiac arrest has not been systematically investigated. In this study, we performed continuous electroencephalography in rats undergoing experimental cardiac arrest and analyzed changes in power density, coherence, directed connectivity, and cross-frequency coupling. We identified a transient surge of synchronous gamma oscillations that occurred within the first 30 s after cardiac arrest and preceded isoelectric electroencephalogram. Gamma oscillations during cardiac arrest were global and highly coherent; moreover, this frequency band exhibited a striking increase in anterior-posterior-directed connectivity and tight phase-coupling to both theta and alpha waves. High-frequency neurophysiological activity in the near-death state exceeded levels found during the conscious waking state. These data demonstrate that the mammalian brain can, albeit paradoxically, generate neural correlates of heightened conscious processing at near-death.
Keywords: consciousness; global hypoxia; global ischemia; near-death experience.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
End-of-life electrical surges.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):E4123. doi: 10.1073/pnas.1315409110. Epub 2013 Oct 18. Proc Natl Acad Sci U S A. 2013. PMID: 24143813 Free PMC article. No abstract available.
-
Surge of neurophysiological activity in the dying brain.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4405. doi: 10.1073/pnas.1316937110. Epub 2013 Nov 6. Proc Natl Acad Sci U S A. 2013. PMID: 24198339 Free PMC article. No abstract available.
-
Reply to Chawla and Seneff: Near-death electrical brain activity in humans and animals requires additional studies.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):E4124. doi: 10.1073/pnas.1316024110. Proc Natl Acad Sci U S A. 2013. PMID: 24344411 Free PMC article. No abstract available.
-
Reply to Greyson et al.: Experimental evidence lays a foundation for a rational understanding of near-death experiences.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4406. doi: 10.1073/pnas.1317358110. Proc Natl Acad Sci U S A. 2013. PMID: 24396876 Free PMC article. No abstract available.
References
-
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98–108. - PubMed
-
- Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187(3):268–283; discussion 284–292. - PubMed
-
- Parnia S, Waller DG, Yeates R, Fenwick P. A qualitative and quantitative study of the incidence, features and aetiology of near death experiences in cardiac arrest survivors. Resuscitation. 2001;48(2):149–156. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

