CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability
- PMID: 23940348
- PMCID: PMC3761631
- DOI: 10.1073/pnas.1221740110
CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability
Abstract
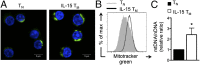
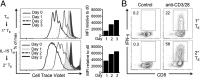
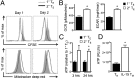
A characteristic of memory T (TM) cells is their ability to mount faster and stronger responses to reinfection than naïve T (TN) cells do in response to an initial infection. However, the mechanisms that allow this rapid recall are not completely understood. We found that CD8 TM cells have more mitochondrial mass than CD8 TN cells and, that upon activation, the resulting secondary effector T (TE) cells proliferate more quickly, produce more cytokines, and maintain greater ATP levels than primary effector T cells. We also found that after activation, TM cells increase oxidative phosphorylation and aerobic glycolysis and sustain this increase to a greater extent than TN cells, suggesting that greater mitochondrial mass in TM cells not only promotes oxidative capacity, but also glycolytic capacity. We show that mitochondrial ATP is essential for the rapid induction of glycolysis in response to activation and the initiation of proliferation of both TN and TM cells. We also found that fatty acid oxidation is needed for TM cells to rapidly respond upon restimulation. Finally, we show that dissociation of the glycolysis enzyme hexokinase from mitochondria impairs proliferation and blocks the rapid induction of glycolysis upon T-cell receptor stimulation in TM cells. Our results demonstrate that greater mitochondrial mass endows TM cells with a bioenergetic advantage that underlies their ability to rapidly recall in response to reinfection.
Keywords: lymphocytes; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- DiSpirito JR, Shen H. Quick to remember, slow to forget: Rapid recall responses of memory CD8+ T cells. Cell Res. 2010;20(1):13–23. - PubMed
-
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47–53. - PubMed
-
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169(7):3760–3770. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

